
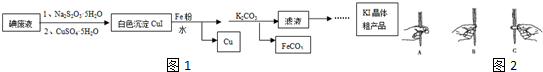
·ÖĪö £Ø1£©øł¾ŻÖŠŗĶµĪ¶ØÓŠ¼ģĀ©”¢Ļ“µÓ”¢ČóĻ“”¢×°Ņŗ”¢µ÷Įć”¢Č”“ż²āŅŗ¼ÓÖøŹ¾¼Į”¢µĪ¶ØµČ²Ł×÷¹ż³Ģ£»
£Ø2£©Ö±½Ó×°Čė±ź×¼ČÜŅŗ£¬±ź×¼ŅŗµÄÅضČĘ«µĶ£»
£Ø3£©øł¾Żc£Ø“ż²ā£©=$\frac{c£Ø±ź×¢£©”ĮV£Ø±ź×¼£©}{V£Ø“ż²ā£©}$·ÖĪö²»µ±²Ł×÷¶ŌV£Ø±ź×¼£©µÄÓ°Ļģ£¬ŅŌ“ĖÅŠ¶ĻÅØ¶ČµÄĪó²ī£»
£Ø4£©×¶ŠĪĘæĻĀµęŅ»ÕÅ°×Ö½Ź¹µĪ¶ØÖÕµćŃÕÉ«±ä»ÆøüĆ÷ĻŌ£¬±ćÓŚ·Ö±ę£»
£Ø5£©ČēČÜŅŗŃÕÉ«±ä»ÆĒŅ°ė·ÖÖÓÄŚ²»±äÉ«£¬æÉĖµĆ÷“ļµ½µĪ¶ØÖÕµć£»
£Ø6£©øł¾Żc£Ø“ż²ā£©=$\frac{c£Ø±ź×¢£©”ĮV£Ø±ź×¼£©}{V£Ø“ż²ā£©}$·ÖĪö²»µ±²Ł×÷¶ŌV£Ø±ź×¼£©µÄÓ°Ļģ£¬ŅŌ“ĖÅŠ¶ĻÅØ¶ČµÄĪó²ī£®
½ā“š ½ā£ŗ£Ø1£©ÖŠŗĶµĪ¶ØÓŠ¼ģĀ©”¢Ļ“µÓ”¢ČóĻ“”¢×°Ņŗ”¢µ÷Įć”¢Č”“ż²āŅŗ¼ÓÖøŹ¾¼Į”¢µĪ¶ØµČ²Ł×÷£¬
¹Ź“š°øĪŖ£ŗBDCEAF£»
£Ø2£©µĪ¶Ø¹ÜÓĆÕōĮóĖ®Ļ“µÓŗó£¬ÄŚ±ŚÓŠŅ»²ćĖ®Ä¤£¬Čē¹ūÖ±½Ó×°Ņŗ»įŹ¹ÅØ¶Č½µµĶ£¬ĖłŅŌ±ŲŠėÓƱź×¼ČÜŅŗČóĻ“µĪ¶Ø¹Ü2-3“Ī£¬
¹Ź“š°øĪŖ£ŗĻ“Č„µĪ¶Ø¹ÜÄŚ±Śø½×ŵÄĖ®£¬·ĄÖ¹½«±ź×¼ŅŗĻ”ŹĶ¶ų²śÉśĪó²ī£»
£Ø3£©×¶ŠĪĘæÓĆÕōĮóĖ®Ļ“µÓŗó£¬Čē¹ūŌŁÓĆ“ż²āŅŗČóĻ“£¬»įŹ¹×¶ŠĪĘæÄŚČÜÖŹµÄĪļÖŹµÄĮæŌö“󣬻įŌģ³ÉV£Ø±ź×¼£©Ę«“ó£¬øł¾Żc£Ø“ż²ā£©=$\frac{c£Ø±ź×¢£©”ĮV£Ø±ź×¼£©}{V£Ø“ż²ā£©}$·ÖĪö£¬²ā¶Ø½į¹ūĘ«øߣ¬
¹Ź“š°øĪŖ£ŗŹ¹²āµĆĪ“ÖŖŅŗµÄÅضČĘ«“ó£»
£Ø4£©Ņņ׶ŠĪĘæĻĀµęŅ»ÕÅ°×Ö½Ź¹µĪ¶ØÖÕµćŃÕÉ«±ä»ÆøüĆ÷ĻŌ£¬±ćÓŚ·Ö±ę£¬
¹Ź“š°øĪŖ£ŗ±ćÓŚ¹Ū²ģČÜŅŗŃÕÉ«±ä»»£¬×¼Č·ÅŠ¶ĻµĪ¶ØÖÕµć£»
£Ø5£©ÓĆ0.1mol•L-1µÄKOH±ź×¼ČÜŅŗµĪ¶ØĪ“ÖŖÅØ¶ČµÄŃĪĖį£¬ÓĆ·ÓĢŖ×÷ÖøŹ¾¼Į£¬ĖłŅŌÖÕµćŹ±ĻÖĻóŹĒµ±ČÜŅŗÓÉĪŽÉ«±äĪŖĒ³ŗģÉ«£¬ĒŅŌŚ°ė·ÖÖÓÄŚ²»ĶŹÉ«£¬
¹Ź“š°øĪŖ£ŗµ±ČÜŅŗÓÉĪŽÉ«±äĪŖĒ³ŗģÉ«£¬ĒŅŌŚ°ė·ÖÖÓÄŚ²»ĶŹÉ«£»
£Ø6£©ČēĖłÓĆKOHŗ¬ÓŠÉŁĮæNaOH£¬Ōņ»įŹ¹½į¹ūĘ«Š”£¬ŅņĪŖĶ¬ÖŹĮæµÄNaOH±ČKOHÖŠŗĶÄÜĮ¦Ē棬ĖłŠčČÜŅŗµÄĢå»żÉŁ£¬ĖłŅŌĘ«Š”£¬
¹Ź“š°øĪŖ£ŗŹ¹²āµĆĪ“ÖŖŅŗÅضČĘ«Š”£®
µćĘĄ ±¾Ģāæ¼²éĪļÖŹµÄŗ¬ĮæµÄ²ā¶Ø£¬ĪŖøßĘµæ¼µć£¬²ąÖŲæ¼²é»ÆѧŹµŃé²Ł×÷ÖŠµÄĪó²ī·ÖĪö£¬ĢāÄæÄѶČÖŠµČ£¬ÕĘĪÕŹµŃéµÄŌĄķ¼°ÕżČ·µÄĪó²ī·ÖĪöŹĒ½āĢāµÄ¹Ų¼ü£¬ŹŌĢāÅąŃųĮĖѧɜĮé»īÓ¦ÓĆĖłŃ§ÖŖŹ¶µÄÄÜĮ¦£®


 53ĢģĢģĮ·ĻµĮŠ“š°ø
53ĢģĢģĮ·ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
| ŅŅ“¼ | 1£¬2-¶žäåŅŅĶé | ŅŅĆŃ | äå | |
| דĢ¬ | ĪŽÉ«ŅŗĢå | ĪŽÉ«ŅŗĢå | ĪŽÉ«ŅŗĢå | ŗģ×ŲÉ«ŅŗĢå |
| ĆÜ¶Č£Øg/mL£© | 0.79 | 2.18 | 0.71 | 3.10 |
| ·Šµć/”ę | 78.5 | 131.4 | 34.6 | 58.8 |
| ČŪµć/”ę | -114.3 | 9.79 | -116.2 | -7.2 |
| Ė®ČÜŠŌ | »ģČÜ | ÄŃČÜ | Ī¢ČÜ | æÉČÜ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ŅŅĻ©”śŅŅ¶ž“¼£ŗCH2ØTCH2$\stackrel{¼Ó³É}{”ś}$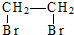 $\stackrel{Č”“ś}{”ś}$ $\stackrel{Č”“ś}{”ś}$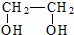 | |
| B£® | äåŅŅĶ锜ŅŅ“¼£ŗCH3CH2Br$\stackrel{ĻūČ„}{”ś}$CH2ØTCH2$\stackrel{¼Ó³É}{”ś}$CH3CH2OH | |
| C£® | 1-ä嶔Ķ锜1£¬3-¶”¶žĻ©£ŗCH3CH2CH2CH2Br$\stackrel{ĻūČ„}{”ś}$CH3CH2CH=CH2$\stackrel{¼Ó³É}{”ś}$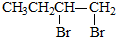 $\stackrel{ĻūČ„}{”ś}$CH2ØTCH-CHØTCH2 $\stackrel{ĻūČ„}{”ś}$CH2ØTCH-CHØTCH2 | |
| D£® | ŅŅĻ©”śŅŅČ²£ŗCH2ØTCH2$\stackrel{¼Ó³É}{”ś}$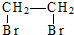 $\stackrel{ĻūČ„}{”ś}$CH”ŌCH $\stackrel{ĻūČ„}{”ś}$CH”ŌCH |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| ŹµŃé | ĪĀ¶Č/”ę | ĘšŹ¼Ź± | Ę½ŗāŹ± | |||
| n£ØCO£©/mol | n£ØH2S£©/mol | n£ØCOS£©/mol | n£ØH2£©/mol | n£ØCO£©/mol | ||
| 1 | 150 | 10.0 | 10.0 | 0 | 0 | 7.0 |
| 2 | 150 | 7.0 | 8.0 | 2.0 | 4.5 | a |
| 3 | 400 | 20.0 | 20.0 | 0 | 0 | 16.0 |
| A£® | ÉĻŹö·“Ó¦ŹĒĪüČČ·“Ó¦ | |
| B£® | ŹµŃé1 “ļĘ½ŗāŹ±£¬CO µÄ×Ŗ»ÆĀŹĪŖ70% | |
| C£® | ŹµŃé2 “ļĘ½ŗāŹ±£¬a£¼7.0 | |
| D£® | ŹµŃé3 “ļĘ½ŗāŗó£¬ŌŁ³äČė1.0molH2£¬K ÖµŌö“ó£¬Ę½ŗāÄęĻņŅĘ¶Æ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | MnO2 | B£® | CO2 | C£® | H2O2 | D£® | æÕĘų |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 25”ꏱ£¬ÓĆ“×ĖįČÜŅŗµĪ¶ØµČÅضČNaOHČÜŅŗÖĮpH=7£¬V£Ø“×Ėį£©£¼V£ØNaOH£© | |
| B£® | Ļ”“×Ėį¼ÓĖ®Ļ”ŹĶ£¬“×ĖįµēĄė³Ģ¶ČŌö“ó£¬ČÜŅŗµÄpH¼õŠ” | |
| C£® | ³£ĪĀĻĀ½«pH=3Ļ”“×ĖįČÜŅŗĻ”ŹĶµ½ŌĢå»żµÄ10±¶ŗó£¬ČÜŅŗµÄpH=4 | |
| D£® | 25”ꏱ£¬amol/LŅ»ŌŖĖįÓėbmol/LNaOHµČĢå»ż»ģŗĻŗó£¬pH=7£¬ĖłµĆ»ģŗĻČÜŅŗÖŠŅ»¶ØÓŠc£ØA-£©=c£ØNA+£© |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com