
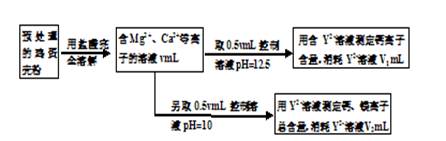

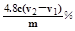 ЃЈ3ЗжЃЉ
ЃЈ3ЗжЃЉ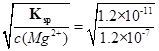 =0.01mol/LЃЌЙЪpHВЛаЁгк12ЃЛ
=0.01mol/LЃЌЙЪpHВЛаЁгк12ЃЛ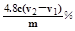


 УћаЃПЮЬУЯЕСаД№АИ
УћаЃПЮЬУЯЕСаД№АИ
| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃКВЛЯъ ЬтаЭЃКЪЕбщЬт

ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃКВЛЯъ ЬтаЭЃКЪЕбщЬт
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃКВЛЯъ ЬтаЭЃКЪЕбщЬт
| ЪЕбщзАжУ | МьбщЦјЬх | ЪдМСУћГЦ | ЪЕбщЯжЯѓ |
| A | SO2 | | |
| B | CO2 | | |
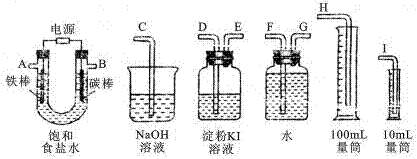
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃКВЛЯъ ЬтаЭЃКЪЕбщЬт
| V/(NaOH)/mL | 0.00 | 10.00 | 18.00 | 19.80 | 19.98 | 20.00 | 20.02 | 20.20 | 22.00 | 40.00 |
| ШмвКpH | 2.87 | 4.74 | 5.70 | 6.74 | 7.74 | 8.72 | 9.70 | 10.70 | 11.70 | 12.50 |
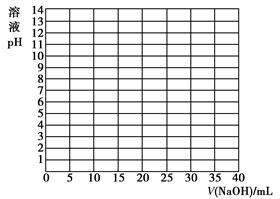
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃКВЛЯъ ЬтаЭЃКЕЅбЁЬт
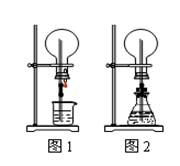
| AЃЎCuКЭЯЁбЮЫс | BЃЎNaHCO3гыNaOHШмвК |
| CЃЎMnO2гыЯЁбЮЫс | DЃЎNa2CO3гыЯЁбЮЫс |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃКВЛЯъ ЬтаЭЃКЕЅбЁЬт
| бЁЯю | ЪЕбщВйзїЁЂЯжЯѓ | НтЪЭЁЂНсТл |
| AЃЎ | ЭЦЌЗХШыХЈСђЫсжаЃЌЮоУїЯдБфЛЏ | ЭдкРфЕФХЈСђЫсжаЛсЗЂЩњЖлЛЏ |
| BЃЎ | ЭљФГТШЛЏЮяШмвКжаЕЮМгАБЫЎЃЌВњЩњАзЩЋГСЕэ | ИУТШЛЏЮяЪЧAlCl3 |
| CЃЎ | НЋ10mlФГpH=3ЕФHAШмвКМгЫЎЯЁЪЭЕН100mlЃЌЫљЕУШмвКpH=3.8 | HAЪЧШѕЫс |
| DЃЎ | ЭљMgCl2ШмвКжаЕЮМгNaOHШмвКЃЌЕїжСpH=9ЪБЃЌПЊЪМГіЯжГСЕэ[вбжЊMg(OH)2ЕФKsp=5.6ЁС10-12] | дШмвКжа c(Mg2+)=5.6ЁС10-2molЁЄL-1 |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃКВЛЯъ ЬтаЭЃКЬюПеЬт

ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃКВЛЯъ ЬтаЭЃКЬюПеЬт
| AЃЎKIШмвК | BЃЎЕэЗлШмвК | CЃЎNaOHШмвК | DЃЎЯЁH2SO4EЃЎТШЫЎ |
ВщПДД№АИКЭНтЮі>>
АйЖШжТаХ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com