
| |||||||||||
(1) |
K2O��2UO3��V2O5��3H2O ����������Ĺؼ�����ȷ�������˵Ļ��ϼۣ�23�Ž���Ԫ�ط�ʧ���Ӵ�8e���ȶ�״ ̬ʱ�ṹΪ�� |
(2) |
H2C2O4,0.51 �����������������ܷ�Ӧʽ֪����Ԫ����VO ������������H2C2O4����ԭ�������Ӧ��Ԫ������Ϊx������ݵ��ӵ�ʧ�غ��У� |


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� | VOSO4 | V2O5 | NH4VO3 | ��VO2��2SO4 |
| �ܽ��� | ���� | ���� | ���� | ���� |

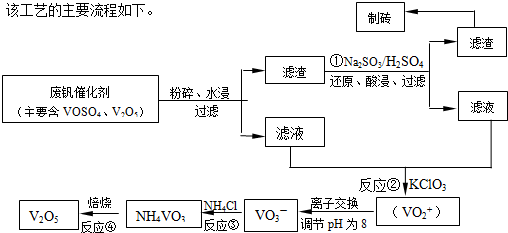
 ����a��c�����Ļ�ѧ����ʽ�ɱ�ʾΪ
����a��c�����Ļ�ѧ����ʽ�ɱ�ʾΪ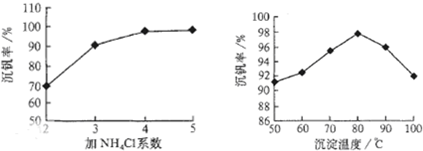
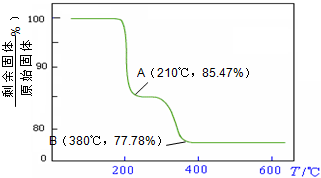 ��NH4VO3�ڷֽ������
��NH4VO3�ڷֽ�������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� | VOSO4 | V2O5 | NH4VO3 | ��VO2��2SO4 |
| �ܽ��� | ���� | ���� | ���� | ���� |
| ||
| ||
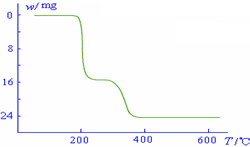 ������������ͼ��ʾ����NH4VO3�ڷֽ������
������������ͼ��ʾ����NH4VO3�ڷֽ�������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
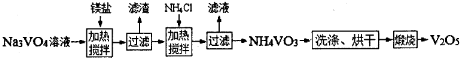
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� | VOSO4 | V2O5 | NH4VO3 | ��VO2��2SO4 |
| �ܽ��� | ���� | ���� | ���� | ���� |
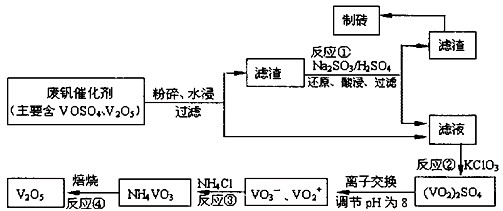
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com