
��13�֣�
��3�֣�����ʵ���������ȷ����
| A����Һʱ����Һ©���²�Һ����¶˷ų����ϲ�Һ����Ͽڵ��� |
| B������ʱ��Ӧʹ�¶ȼ�ˮ����������ƿ֧�ܿ� |
| C�������ᾧʱӦ����Һֱ������ |
| D�������Ǹ�ʴ��ҩƷӦ����������ƽ���̵ij���ֽ�ϣ��������������ƽ���̵ij���ֽ�� |
| ʵ�鲽�� | ���� | ���� |
| �ٷֱ�ȡ�����2mol/L���������Թ��� �� | ��Ӧ����Mg��Fe��Cu��Ӧ���� | ��������Խ���ã���Ӧ ����Խ�� |


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
| 11200 |
| V |
| 11200 |
| V |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


| ||
| ||
| 11200m |
| V |
| 11200m |
| V |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�ش��������⡣
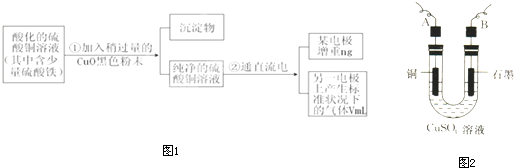
(1)����CuO�������� _______________________________ ��
(2)����������õIJ�����������ͼ��ʾ����A��B�ֱ���ֱ����Դ�ļ�_______��_______��(���������)
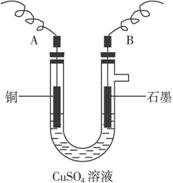
(3)��ʼ����U�ι��п��Թ۲쵽�������У�________________���������ӷ���ʽΪ________________________________________________��
(4)����ʵ������б�Ҫ����_______ (����ĸ)��
A.�������ǰ�缫������
B.���缫�ں�ɳ���ǰ������������ˮ��ϴ
C.���µ���缫��������ͭ������ϴ������
D.�����ɳ��صIJ����б��밴����ɳ����ٺ���ٳ���������
E.���п������ڵ�����£���ɵ缫�����õ��º�ɵķ���
(5)ͭ�����ԭ������Ϊ__________________ (�ô���m��V�ļ���ʽ��ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��12�֣�
��֪pHΪ4��5�������£�Cu2+������ˮ�⣬��Fe3+������ȫˮ�⡣ijѧ���õ�ⴿ����CuSO4��Һ�ķ����������ݵ缫������Cu��������n���Լ��缫�ϲ�������������V mL ��״�������ⶨCu�����ԭ���������������£�
�ش��������⣺
��1������CuO�������� ��
��2������������õIJ���������ͼ��ʾ����A��ֱ����Դ�� �����������������
��3����ʼ����U�ι��п��Թ۲쵽�������У�
������ ��������������������������
�������ӷ���ʽ���ܷ�Ӧ��Ϊ
�������������������������������� ��
��4������ʵ������б�Ҫ���� ����д��ĸ����
��A���������ǰ�ĵ缫��������
��B�����缫�ں�ɳ���ǰ������������ˮ��ϴ��
��C�����µ���缫��������ͭ������ϴ��������
��D�������ɳ��صIJ����б��밴����ɡ��������ٺ�ɡ��ٳ��������У�
��E�����п������ڵ�����£���ɵ缫�����õ��º�ɵķ�����
��5��ͭ�����ԭ������Ϊ ���ô���m��V�ļ���ʽ��ʾ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ɽ��ʡ�߶���ѧ�ڽ��Բ��Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
��12�֣���֪pHΪ4��5�������£�Cu2+������ˮ�⣬��Fe3+������ȫˮ�⡣ijѧ���õ�ⴿ����CuSO4��Һ�ķ����������ݵ缫������Cu��������n���Լ��缫�ϲ�������������V mL ��״�������ⶨCu�����ԭ���������������£�
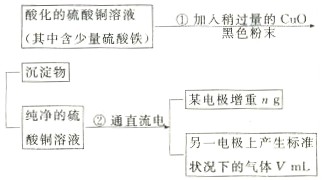
�ش��������⣺
��1������CuO�������� ��
��2������������õIJ�����������ͼ��ʾ����A��B�ֱ���ֱ����Դ�� �� �����������������
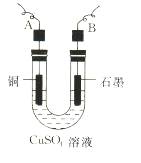
��3����ʼ����U�ι��п��Թ۲쵽�������У� ���������ӷ���ʽΪ ��
��4������ʵ������б�Ҫ���� ����д��ĸ����
��A���������ǰ�ĵ缫����������B�����缫�ں�ɳ���ǰ������������ˮ��ϴ����C�����µ���缫��������ͭ������ϴ����������D�������ɳ��صIJ����б��밴����ɡ��������ٺ�ɡ��ٳ��������У���E�����п������ڵ�����£���ɵ缫�����õ��º�ɵķ�����
��5��ͭ�����ԭ������Ϊ ���ô���m��V�ļ���ʽ��ʾ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com