
�Լ�������(������ͭ)������(������Fe3��)��Ӧ��ȡ���������������£�
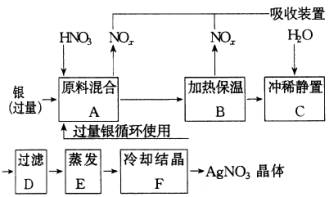
�����������裬���������գ�
(1)�ܽ���������Ӧ����________����(����Ũ������ϡ��)��ԭ����________��
A�����ٹ����в���NOx������
B������ԭ��������������
C����ʡ�������ʵ�����
(2)����B���ȱ��µ�������________��
A�������ڼӿ췴Ӧ���ʣ�
B��������δ��Ӧ������ӷ���
C�������������ַ�Ӧ��������Һ��[H+]��
(3)����C��Ϊ�˳�ȥFe3+��Cu2+�����ʡ���ϡ����ʱ�����Ļ�ѧ��Ӧ��_ ___���� ___ _������������������������ �������ij����ﻯѧʽ��___������������ _____��
A���û���Ӧ��
B��ˮ�ⷴӦ��
C��������ԭ��Ӧ��
(1)ϡ��a��c��(2)a��c��(3)b��Fe(OH)3��Cu(OH)2�� |
(1)��Cu+4HNO3(Ũ)=Cu(NO3)2+2NO2��+2H2O��3Cu+8HNO3(ϡ)=3Cu(NO3)2+2NO��+4H2O��������������Ӧʽ��֪��Ϊa��c�� (3)�У�����Fe3+��Cu2+��ˮ������ǿ����ʹ�������������£����Ƕ����Է���ˮ�⡣��Һϡ�ͺ�pH����������Fe3+��Cu2+��ˮ�⣬����Fe(OH)3��Cu(OH)2�ij����������˺õ�������AgNO3��Һ��
|


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�갲��ʡ������һѧ�����п��Ի�ѧ�Ծ� ���ͣ�ʵ����
��14�֣�ÿ��2�֣�ij�Լ���������������ͭ�������ᣨ��Fe3+����Ӧ��ȡ���������������£�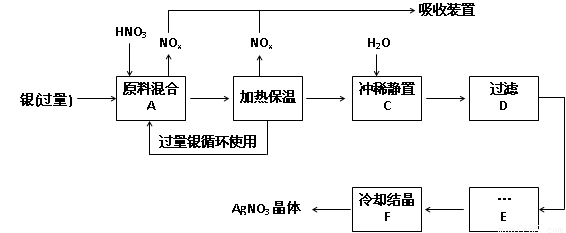
��1����ҵ��һ��ѡ���е�Ũ�ȵ����������Ӧ����ȡ�������������±��ո���ա�
|
|
�ŵ� |
ȱ�� |
|
ʹ��Ũ���� |
��Ӧ���ʿ� |
��Ľϴ���NOx�����϶� |
|
ʹ��ϡ���� |
|
|
��2������B���ȱ��µ������� ��
a�� �����ڼӿ췴Ӧ����
b��������δ��Ӧ������ӷ�
c�������������ַ�Ӧ��������Һ��H+��Ũ��
��3������C��Ϊ�˳�ȥFe3+��Cu2+�����ʣ���ϡʱ����������ԭ���� ��
��4������C�м�ˮ����Ӧ������������������ˮ���Ժ���������ɵIJ���Ӱ���ǣ�
��
��5������E���еIJ����� ��
��6���Ƶõ��������к�����������ͭ��ͨ����ȥ����ͭ�ķ������ڲ���E֮ǰ���������Ƶ�Ag2O��ʹCu2+ת��ΪCu(OH)2��������Ӧ����˳�ȥ���÷�Ӧ�Ļ�ѧ����ʽΪ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��0112 ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
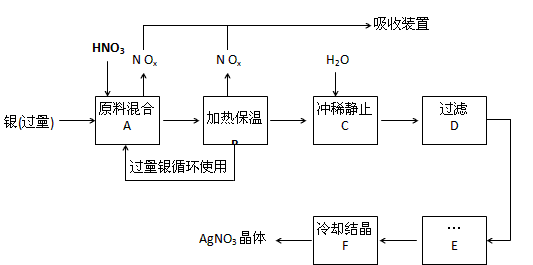
��12�֣�ij�Լ���������������ͭ�������ᣨ��Fe3+����Ӧ��ȡ���������������£�
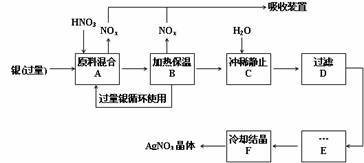
��1����ҵ��һ��ѡ���е�Ũ�ȵ����������Ӧ����ȡ�������������±��ո���ա�
| �ŵ� | ȱ�� |
ʹ��Ũ���� | ��Ӧ���ʿ� | ��Ľϴ���NOx�����϶� |
ʹ��ϡ���� |
|
|
��2������B���ȱ��µ������� ��
a�������ڼӿ췴Ӧ����
b��������δ��Ӧ������ӷ�
c�������������ַ�Ӧ��������Һ��H+��Ũ��
��3������C��Ϊ�˳�ȥFe3+��Cu2+�����ʣ���ϡʱ����������ԭ���� ��
��4������C�м�ˮ����Ӧ������������������ˮ���Ժ���������ɵIJ���Ӱ���ǣ� ��
��5������E���еIJ����� ��
��6���Ƶõ��������к�����������ͭ��ͨ����ȥ����ͭ�ķ������ڲ���E֮ǰ���������Ƶ�Ag2O��ʹCu2+ת��ΪCu(OH)2��������Ӧ����˳�ȥ���÷�Ӧ�Ļ�ѧ����ʽΪ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��14�֣�ÿ��2�֣�ij�Լ���������������ͭ�������ᣨ��Fe3+����Ӧ��ȡ���������������£�
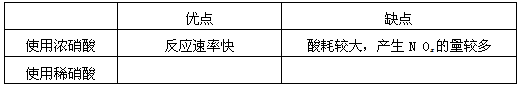
��1����ҵ��һ��ѡ���е�Ũ�ȵ����������Ӧ����ȡ�������������±��ո���ա�
|
| �ŵ� | ȱ�� |
| ʹ��Ũ���� | ��Ӧ���ʿ� | ��Ľϴ���NOx�����϶� |
| ʹ��ϡ���� |
|
|
��2������B���ȱ��µ������� ��
a�� �����ڼӿ췴Ӧ����
b��������δ��Ӧ������ӷ�
c�������������ַ�Ӧ��������Һ��H+��Ũ��
��3������C��Ϊ�˳�ȥFe3+��Cu2+�����ʣ���ϡʱ����������ԭ���� ��
��4������C�м�ˮ����Ӧ������������������ˮ���Ժ���������ɵIJ���Ӱ���ǣ�
��
��5������E���еIJ����� ��
��6���Ƶõ��������к�����������ͭ��ͨ����ȥ����ͭ�ķ������ڲ���E֮ǰ���������Ƶ�Ag2O��ʹCu2+ת��ΪCu(OH)2��������Ӧ����˳�ȥ���÷�Ӧ�Ļ�ѧ����ʽΪ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�찲��ʡ�����ѧ������һѧ�����п��Ի�ѧ�Ծ� ���ͣ�ʵ����
��14�֣�ÿ��2�֣�ij�Լ���������������ͭ�������ᣨ��Fe3+����Ӧ��ȡ���������������£�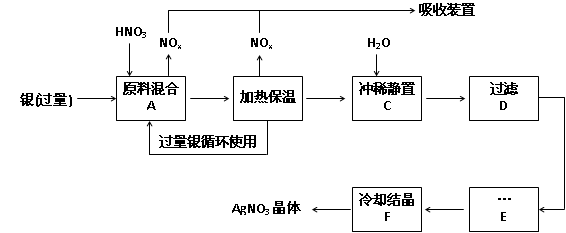
��1����ҵ��һ��ѡ���е�Ũ�ȵ����������Ӧ����ȡ�������������±��ո���ա�
| | �ŵ� | ȱ�� |
| ʹ��Ũ���� | ��Ӧ���ʿ� | ��Ľϴ���NOx�����϶� |
| ʹ��ϡ���� | | |
 ��
�� ��
�� Ӧ�Ļ�ѧ����ʽΪ�� ��
Ӧ�Ļ�ѧ����ʽΪ�� ���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com