

���� ��1������Ũ������������Խ�Ϸ�Ӧ���ʵ��ټ���98%����������Ŀ���ǣ��ӿ����ˮ������ʣ�������������ã���
��2����ˮ�������ۻ�����������Һ�����ۣ������ˮû�б仯��
��3�������ܵ������������������������������ã���������ˮ������Ϊ������
��4���л�������װ���ܷ�ֹ������
��5��������л�ԭ�ԣ������ܽ�һ������C6H12O6��H2C2O4��
��6��NO�е�Ԫ��Ϊ+2�ۣ�NO2�е�Ԫ��Ϊ+4�ۣ��ڼ��������£�������̬���з�Ӧ���ŵ㣺���HNO3�����ʣ���ѭ��ʹ�õ�������� ȱ�㣺NOx������������ղ���ȫ��
��7���������Ϊ�Ϻ�ɫ�������в���Ҫ��ָʾ���������������£�������������ܺͲ��ᷢ��������ԭ��Ӧ���ɶ��������ӡ�������̼��ˮ�����ݷ�Ӧ���㣮
��� �⣺��1��Ũ�������ǿ�����ԡ���ˮ�Ժ���ˮ�ԣ�����ʵ���ǽ�C6H12O6���������������Ʊ����ᣬŨ������������Ũ������ˮ�����������ɲ���ķ����ƶ�������ʵ��ټ���98%����������Ŀ���ǣ��ӿ����ˮ������ʣ�������������ã���
�ʴ�Ϊ���ӿ����ˮ������ʣ�������������ã���
��2�������������ɫ�����Ѿ�ˮ��ĵ�����Һ�еμӼ��ε�Һ����Һ����ɫ����֤������û����ȫˮ�⣻��Һ������ɫ����֤��������ȫˮ�⣬
�ʴ�Ϊ����ˮ��
��3�����ܵ������������������������������ã�����Ч������Ч���ã�����ˮ�Ľ�����a��b����
�ʴ�Ϊ��a��
��4��װ��B�������Ƿ�ֹ����װ�ú�����װ�ü䷢����������ȫƿ�����ã�
�ʴ�Ϊ������ȫƿ��
��5������Ϊ65%HNO3��98%H2SO4�Ļ��Һ�����Һ����ˮ���ȣ��¶ȸ��ܼӿ컯ѧ��Ӧ�������ܽ�һ������H2C2O4�ɶ�����̼��
�ʴ�Ϊ������Ũ�ȹ�����C6H12O6 ��H2C2O4��һ����������
��6��������ӦΪ���з�Ӧ������NԪ�صĻ��ϼۿ�֪Ӧ����NaNO2����Ӧ�ķ���ʽΪNO+NO2+2NaOH=2NaNO2+H2O���ú������ĸҺ�����յ������������������ظ�ʹ�ã����HNO3�����ʣ���Ҳ��������ղ���֣���ɻ�����Ⱦ��
�ʴ�Ϊ��NO2+NO+2NaOH=2NaNO2+H2O�����HNO3�����ʣ�NOx������������ղ���ȫ��
��7�����������ҺΪ�Ϻ�ɫ�����ﵽ�ζ��յ�ʱ���ٵ�����������Һʱ������ɫ������ȥ�������ƣ�Na2C2O4������ϡ�����У�Ȼ�������Ը��������Һ���еζ������ӷ���ʽΪ��2MnO4-+5C2O42-+16H+=2Mn2++10CO2��+8H2O��n��KMnO4��=0.016L��0.0200mol•L-1=3.2��10-3mol�����ݷ���ʽ�ɵã�
2MnO4-+5C2O42-+16H+=2Mn2++10CO2��+8H2O��
2 5
3.2��10-3mol 8��10-3mol
��Ʒ�ж�ˮ�ϲ��������Ϊm=8��10-3mol��126g/mol=8��126��10-3g=1.008g��
��ò��ᾧ����Ʒ�ж�ˮ�ϲ������������Ϊ$\frac{1.008g}{1.2g}$��100%=84%��
�ʴ�Ϊ����ɫ������ɫ��84%��
���� ������Ҫ�����˲������ȡʵ�飬ע�����ʵ���ԭ������������������ԭ�����ǽ��Ĺؼ���Ҫ��߱�һ�������۷��������ͼ������������������Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 24.4 g | B�� | 13.2 g | C�� | 12.5 g | D�� | 11.2 g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
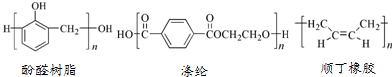
| A�� | ˳�������ںͷ�ȩ��֬��������Ȼ�߷��Ӳ��� | |
| B�� | ˳���ĵ����뷴-2-��ϩ��Ϊͬ���칹�� | |
| C�� | �����ǶԱ���������Ҷ���ͨ�����۷�Ӧ�õ��� | |
| D�� | ��ȩ��֬�ĵ����DZ��Ӻͼ״� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | n��CH3COOH�� | B�� | c��H+�� | C�� | c��H+��•c��OH-�� | D�� | $\frac{c��{H}^{+}��}{c��C{H}_{3}COOH��}$ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ع��;������������������� | |
| B�� | �������Ż������ĭ�������� | |
| C�� | ���ࡢ��֬�������ʾ�Ϊ�߷��ӻ����� | |
| D�� | ������������ķ�ɢ�����Ӳ���ͨ����ֽ��϶ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���³�ѹ�£�78gNa2O2�к��е�����������NA | |
| B�� | m g ${\;}_{Z}^{A}$Xn-��������������Z-n��mNA/A | |
| C�� | 1L0.1mol/LFe��OH��3�����к���Fe��OH��3������Ϊ0.1NA | |
| D�� | H2SO4��Ħ������Ϊ98 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��Ƭ���ڹ���������������Һ��Al+4OH-�TAlO2-+2H2O | |
| B�� | 0.01mol/LNH4Al��SO4��2��Һ��0.02mol/LBa��OH��2��Һ�������ϣ�Al3++2SO42-+2Ba2++4OH-�T2BaSO4��+AlO2-+2H2O | |
| C�� | ��Na2SiO3��Һ��ͨ�����CO2��SiO32-+CO2+H2O�TH2SiO3��+CO32- | |
| D�� | ��CuSO4��Һ�м���Na2O2��2Na2O2+2Cu2++2H2O�T4Na++2Cu��OH��2��+O2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���ʱ�����ƽ������ӵ�Դ����������������Ӧ | |
| B�� | ��ҵ�ϵ��NaClˮ��Һ�Ʊ��ƣ��������̬Al2O3�Ʊ�Al | |
| C�� | ��������բ����ֱ����Դ�ĸ��������������������������������� | |
| D�� | ��⾫��ͭʱ�����Һѡ�������ữ������ͭ�����������У�Cu2+Ũ�Ƚ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | SO3 | B�� | BaSO4 | C�� | Cl2 | D�� | CH3COOH |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com