
.����ʵ����Ƽ����Ӧ�����ӷ���ʽ����ȷ����( )
A����FeCl3��Һ��ʴͭ��·�壺Cu + 2Fe3+ �� Cu2+ + 2Fe2+
B��Na2O2��H2O��Ӧ�Ʊ�O2 ��Na2O2 + H2O �� 2Na+ + 2OH�� + O2��
C������������ˮ�Ʊ������Cl2 + H2O �� 2H+ + Cl�� + ClO��
D����Ũ�����ữ��KMnO4��Һ��H2O2��Ӧ��֤��H2O2���л�ԭ�ԣ�
2MnO + 6H+ + 5H2O2 �� 2Mn2+ + 5O2�� + 8H2O
��֪ʶ�㡿���ӷ���ʽ�������ж� B1 B3
���𰸽�����A ������A��������ʵ�������غ��ϵ����A��ȷ��B���������غ㣬��B����C��������Ϊ�������Ӧ������ѧʽ����C����D��������Ũ�����ữKMnO4��Һ��KMnO4�ὫŨ������������D����
�ʴ�ѡA
��˼·�㲦�����⿼�������ӷ���ʽ�������жϣ�ע��ǿ������ʵ����������Բ�����Ũ�����ữKMnO4��Һ��KMnO4�ὫŨ����������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
д����CH2ClCH2CH2CH2OHΪԭ���Ʊ�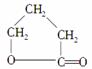 �ĸ�����Ӧ����ʽ(��Ҫ�����Լ���ѡ)��
�ĸ�����Ӧ����ʽ(��Ҫ�����Լ���ѡ)��
��________________________________________________________________________
________________________________________________________________________��
��________________________________________________________________________
________________________________________________________________________��
��________________________________________________________________________
________________________________________________________________________��
��________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ӷ���ʽ��ȷ����
A. ������NaOH��Һ��Al+2OH—=AlO2—+H2��
B. ͭ����ϡ���3Cu+ 8H+ +2NO3—=3Cu2+ +2NO�� + 4H2O
C. ̼��þ�еμ�ϡ���CO32—+2H+ =CO2�� + H2O
D. ϡ�����еμ�����������Һ��H++ OH—=H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���б�ʾ��ѧ��Ӧ�����ӷ���ʽ��������ȷ����
A��NaAlO2��Һ�еμӹ������AlO2��+H2O+H��= AI(OH)3
B������ͨ����ˮ�У�Cl2+H2O 2H��+Cl�� +ClO��
2H��+Cl�� +ClO��
C����������Һ��ͨ��������CO2��2C6H5O��+CO2+H2O =2C6H5OH +CO32��
D��FeCl3��Һ��Cu��Ӧ��2Fe3++Cu = 2Fe2++ Cu2+
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и���������ָ����Һ���ܴ����������
A.�����£�c(H+)/c(OH-)=1��10��12����Һ:K+��AlO2����CO32����Na+
B.ij��Һ�п��ܴ�������:Fe3����K����HCO3����SO42��
C.ʹpH��ֽ��������Һ��:NH4����Na����SO42����Cl��
D.������Һ��:K����Al3����I����ClO����NO3��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ҵ��ˮ�п��ܺ������¼����������ӣ�
| ������ | Fe3+��Al3+��Fe2+��Ba2+��Na+ |
| ������ | Cl����CO32����NO3����SO42����SiO32�� |
�ֶԸ÷�ˮ��Ʒ���������о���
�����Թ��еμ�Ũ���ᣬ����������ɫ�������ɣ�����������������Ϊ����ɫ��
����������õ���Һ�м���BaCl2��Һ���а�ɫ�������ɡ�
����������õ���Һ�м��������NaOH��Һ���к��ɫ�������ɡ����˺���������Һ��ͨ�������CO2���壬�а�ɫ��״�������ɡ�
��������ʵ�飬�ش��������⣺
��1���÷�ˮ��һ�����е���������������������һ�������е������������������� ��
��2��д�����������Һ��ͨ�������CO2�������ɰ�ɫ��״���������ӷ���ʽ��ֻд��һ���������������������� ��
��3����֪�����ۿ��Գ�ȥ��ˮ�е�һ�������ӣ�X������������Һ��pHΪ10.7���ң��ټ������ۣ���ȥX���ӵ�ͬʱ���������͵������������Ϊ1﹕4��������з�Ӧ�����ӷ���ʽ����ƽ��(X�þ�������ӷ��ű�ʾ)
Al���� +�� X +�� OH������AlO2��+�� NH3�� +�� N2�� +����������������
�÷�Ӧ�Ļ�ԭ�������������� ������ȥ0.2mol X���ӣ�Ҫ���������������� g��
��4��������ˮ�е���Ԫ����ȫת����Fe3+����ʱ���c(Fe3+)��1.0��10��2mol·L��1��Ҫ�뽫��ת��ΪFe(OH)3��������ȥ����Ӧ������ҺpH���ٴ������������������������� ������֪������Fe(OH)3��Ksp��1.0��10��38��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijǿ������ҺX����Ba2����Al3����NH ��Fe2����Fe3����CO
��Fe2����Fe3����CO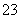 ��SO
��SO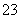 ��SO
��SO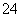 ��Cl����NO
��Cl����NO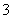 �е�һ�ֻ��֣�ȡ����Һ����ʵ�飬ʵ���������£�
�е�һ�ֻ��֣�ȡ����Һ����ʵ�飬ʵ���������£�
����������Ϣ���ش��������⣺
(1)��ҺX�г�H����϶����е�������_____________________________________��
(2)д���й����ӷ���ʽ��
�����������A__________________;��������ɳ���I__________________��
(3)����ⶨA��F��I��Ϊ0.01 mol,10 mL X��Һ��n(H��)��0.04 mol��������C���ʵ���0.07 mol����˵������Һ����ȷ���������Ӵ��ڵ�������___________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��10L�����ܱ������г���X��g����Y��g����������ӦX��g��+Y��g�� M(g)+N(g)
M(g)+N(g)
����ʵ���������±�:
| ʵ���� | �¶�/�� | ��ʼʱ���ʵ���/mol | ƽ��ʱ���ʵ���/mol | |
| n��X�� | n��Y�� | n��M�� | ||
| �� | 700 | 0.40 | 0.10 | 0.090 |
| �� | 800 | 0.10 | 0.40 | 0.080 |
| �� | 800 | 0.20 | 0.30 | a |
| �� | 900 | 0.10 | 0.15 | b |
�ش���������:
(1)ʵ����У���5minʱ��ã�n(M)=0.050mo1, ��0��5minʱ���ڣ���N��ʾ��ƽ����Ӧ����Ϊ________��
(2)ʵ���ƽ�ⳣ��Ϊ___________��������ӦΪ____________��Ӧ(����ȡ����ȡ�)
(3)��˵��������Ӧһ���ﵽƽ���������_______________________��
A. c(Y)= c(N) B.ƽ�����������ٱ仯
C. v��(X) =v��(M) D.�¶Ⱥ�ѹǿһ��ʱ�����������ܶȲ��ٱ仯
(4)ʵ����У��ﵽƽ��ʱ��X��ת����Ϊ_________________________��
(5)ʵ��ۡ����У��ﵽƽ��ʱ��a��b�Ĺ�ϵΪ__________(��ѡ��)�������ԭ��________________��
A. a>2b B. a=2b C.b<a<2b D.a<b
(6)ͼ��ʵ�����c(M)��ʱ��仯������ͼ������ͼ�л���ʵ�����c(M)��ʱ��仯������ͼ��
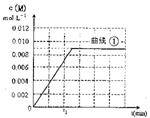
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com