
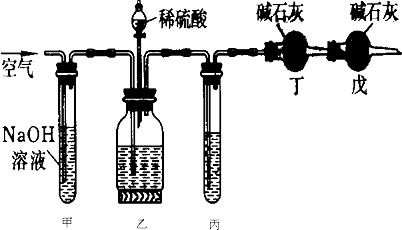
��ҵ�����Ĵ����У�������������![]() �����ʣ���ͼ�Dzⶨ��Ʒ��
�����ʣ���ͼ�Dzⶨ��Ʒ��![]() ����������ʵ��װ�ã�ʵ�����������
����������ʵ��װ�ã�ʵ�����������
(A)�ڸ������������ʯ�ң�����Ϊmg
(B)ȡng��Ʒװ����ƿ��
(C)���װ�õ�������
(D)����������������ӣ��ٳƸ��������Ϊwg
(E)�ر�ֹˮ��
(F)��ֹˮ��
(G)��������ϡ![]() �����ٲ�������Ϊֹ
�����ٲ�������Ϊֹ
(H)�����������������
����ȷ�IJ���˳����(��д����)��C��________��F��________��E��________��G��________��H��
�ڲ�������D�У�Ҫ����������������ӣ����������������________��װ��A��������________��װ��B��������________���ۼ�����Һ��![]() ���������ļ���ʽΪ________������ȥ��װ��A���ⶨ�����________����ȥ��װ��B���ⶨ�����________(�ƫ����ƫС������Ӱ�족)��
���������ļ���ʽΪ________������ȥ��װ��A���ⶨ�����________����ȥ��װ��B���ⶨ�����________(�ƫ����ƫС������Ӱ�족)��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| 106W3 |
| 197W1 |
| 106W3 |
| 197W1 |

| 53(W3-W1) |
| 22W2 |
| 53(W3-W1) |
| 22W2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
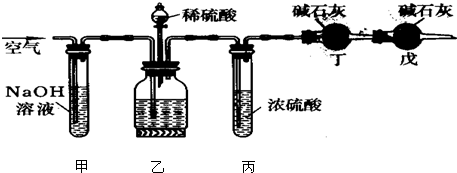 ��ҵ�����Ĵ����г�����������NaCl���ʣ�ijУ�о���ѧϰ�С��Ϊ�˲ⶨ������д����������������ʹ����ͼʵ��װ�ã�˵�������Ӽ��ҵ���Ƥ�������п��ƣ����Ȳⶨһ��������Ʒ���ᷴӦ�ų�������̼���������ټ��������д��������������
��ҵ�����Ĵ����г�����������NaCl���ʣ�ijУ�о���ѧϰ�С��Ϊ�˲ⶨ������д����������������ʹ����ͼʵ��װ�ã�˵�������Ӽ��ҵ���Ƥ�������п��ƣ����Ȳⶨһ��������Ʒ���ᷴӦ�ų�������̼���������ټ��������д��������������| 53(w-m) |
| 22n |
| 53(w-m) |
| 22n |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��ҵ�����Ĵ����г���������NaCl���ʣ�Ϊ�ⶨij������Ʒ�Ĵ��ȣ���ѧ����С�����������ʵ�鷽����
��ҵ�����Ĵ����г���������NaCl���ʣ�Ϊ�ⶨij������Ʒ�Ĵ��ȣ���ѧ����С�����������ʵ�鷽����| 53(W3-W1) |
| 22W2 |
| 53(W3-W1) |
| 22W2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com