
���ж������������������N2O3�ܲ��ȶ�����Һ��������д�����NO��NO2�������NOת����NO2�Ĺ����м���û��N2O3���ɡ�������Ҳ���ȶ�����������������Ҳ��ֽ⡣����������һ���°����ʣ��������Ի�������������ȶ��ģ��ữ���������⻯�����ɵ��NO���塣
��1��д��������ֽ�Ļ�ѧ����ʽ ��
��2��д��������Һ���������ƺ͵⻯�ط�Ӧ��ȡһ�����������ӷ���ʽ
��3���ڸ��������������°����²������������������м���ϡ���ᣬƬ�̺��ټ���⻯����Һ�������Ƶõ������ƽ�������� 30������ڡ�С�ڻ���ڡ�����
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
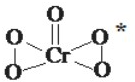 ��1����Ԫ�������Ԫ�ؾ������������γɻ����������࣮ܶ
��1����Ԫ�������Ԫ�ؾ������������γɻ����������࣮ܶ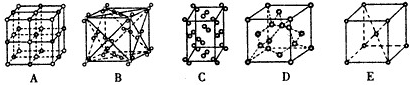
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�꽭��ʡ������ѧ��һ�ڶ�ѧ�����п��Ի�ѧ��ʵ��ࣩ���� ���ͣ������
��12�֣�
A.ԭ������ͬ������������ͬ�ķ��ӣ�����Ϊ�ȵ����塣
��.��֪A��B��C��D��E���ַ�������ԭ�ӵ���Ŀ����Ϊ1��2��3��6��6���Ҷ�����18�����ӣ���֪B��C��D��������Ԫ�ص�ԭ����ɣ���D����������ԭ�Ӹ�����Ϊ1 :2��
��ش�
��1�����A���ӵ�ԭ�ӵ�Ԫ�ط����� ����֪E���ж����л��E���ۡ��е��CH4���ۡ��е�ߣ�����Ҫԭ����____________________________________��
��2��C������ṹ�� ____ �Σ��÷������� ���ӣ�����ԡ��Ǽ��ԡ�����
��3������пɳ���������������D��Ϊȼ�Ϸ�Ӧ���ɵ�����ˮ���÷�Ӧ�Ļ�ѧ����ʽΪ_______ __________________��������Ҫд��Ӧ������
��.CO��N2��Ϊ�ȵ����塣
��4��CO���ܼ��ܴ���N2���ܼ��ܣ���CO��N2���ײμӻ�ѧ��Ӧ��
�����±����ݣ�˵��CO��N2���õ�ԭ����____________________________________��
| | | A��B | A=B | A��B |
| CO | ���ܣ�kJ/mol�� | 357.7 | 798.9 | 1071.9 |
| ���ܲ�ֵkJ/mol�� | 441.2 273 | |||
| N2 | ���ܣ�kJ/mol�� | 154.8 | 418.4 | 941.7 |
| ���ܲ�ֵkJ/mol�� | 263.6 523.3 | |||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ������ʡ�����и�����ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ������
���ж������������N2O5��һ�������������������ʺ��Ʊ��յ����ǵĹ�ע��
��1��һ���¶��£��ں����ܱ�������N2O5�ɷ������з�Ӧ��2N2O5(g)
 4NO2(g)��O2(g) ���SH��0
4NO2(g)��O2(g) ���SH��0
�ٷ�Ӧ�ﵽƽ�������ͨ��һ�����������N2O5��ת���ʽ�___�����������С���������䡱����
���±�Ϊ��Ӧ��T1�¶��µIJ���ʵ�����ݣ�
|
t/s |
0 |
500 |
1000 |
|
c(N2O5)/mol��L��1 |
5.00 |
3.52 |
2.48 |
��500s��N2O5�ķֽ�����Ϊ______________��
��һ���¶��£���2L�����ܱ������м���2mol
N2O5���ﵽƽ��ʱ�������ѹǿΪԭ����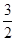 ��(������NO2�ۺϳ�N2O4)����N2O5��ת����a1�� �����¶��·�Ӧ��ƽ�ⳣ��K��_______��
��(������NO2�ۺϳ�N2O4)����N2O5��ת����a1�� �����¶��·�Ӧ��ƽ�ⳣ��K��_______��
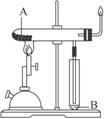
��2����ͼ��ʾװ�ÿ������Ʊ�N2O5����N2O5�ڵ��ص�__________�����ɣ���缫��ӦʽΪ_________________________________��

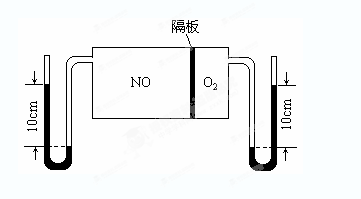
��3����ͼ��V(NO):V(O2)��3:1������ȥNO��O2֮��ĸ����NO��O2��Ӧ���NO2������NO2�ۺϳ�N2O4��N2O4��ʱΪ��̬��������ϵ�ﵽƽ���U��ëϸ�����˹���߶Ȳ��ɷ�Ӧǰ10cm��Ϊ7.1cm�������¶Ȳ��䣬�Ҹ��弰U��ëϸ�ܵ���������Բ��ƣ���ʱ��������ƽ����Է�������Ϊ__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com