
¹¤ŅµÉĻÉś²śøßĀČĖį£Ø·Šµć£ŗ90oC£©Ź±»¹Ķ¬Ź±Éś²śĮĖŃĒĀČĖįÄĘ£¬Ę乤ŅÕĮ÷³ĢČēĻĀ£ŗ
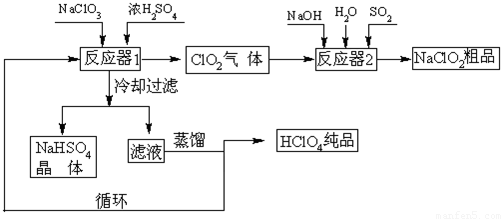
£Ø1£©ĄäČ“¹żĀĖµÄÄæµÄ ”£
£Ø2£©ĶØČė·“Ó¦Ę÷2µÄSO2×÷ÓĆŹĒ £»·“Ó¦Ę÷2ÖŠ·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ ”£
£Ø3£©Ń»·Ź¹ÓƵÄĪļÖŹŹĒ ”£
£Ø4£©æÉŅŌĶعżÕōĮóĀĖŅŗµÄ·½·ØµĆµ½øßĀČĖįµÄŌŅņæÉÄÜŹĒ ”£
£Ø5£©Ķعżµē½āNaClO3Ė®ČÜŅŗµÄ·½·ØŅ²æÉŅŌÖʱøNaClO4£¬½ų¶ųæÉŅŌÖʱøHClO4£¬Š“³öŃō¼«µÄµē¼«·“Ó¦Ź½ ”£
£Ø1£© ½µµĶNaHSO4µÄČܽā¶Č²¢·ÖĄė³öNaHSO4¾§Ģå£Ø2·Ö£©
£Ø2£©¶žŃõ»ÆĮņ×÷ĪŖ»¹Ō¼Į°ŃClO2»¹ŌĪŖNaClO2”¢£Ø2·Ö£©
2ClO2£«SO2£«4OH££½2ClO2££«SO42££«2H2O£»£Ø4·Ö£©
£Ø3£©H2SO4£Ø2·Ö£©
£Ø4£©øßĀČĖįµÄ·Šµć±Č½ĻµĶ£¬ČŻŅדÓČÜŅŗÖŠŅŻ³ö£»£Ø2·Ö£©
£Ø5£© Ńō¼«·“Ó¦Ź½ H2O+ClO3”Ŗ-2e”Ŗ= ClO4”Ŗ + 2H+ ”££Ø4·Ö£©
”¾½āĪö”æĀŌ


 ѧ¶ųÓÅĻĪ½Ó½Ģ²ÄÄĻ¾©“óѧ³ö°ęÉēĻµĮŠ“š°ø
ѧ¶ųÓÅĻĪ½Ó½Ģ²ÄÄĻ¾©“óѧ³ö°ęÉēĻµĮŠ“š°ø Š”ѧæĪĢĆ×÷ŅµĻµĮŠ“š°ø
Š”ѧæĪĢĆ×÷ŅµĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 ¹¤ŅµÉĻÉś²śøßĀČĖį£Ø·Šµć£ŗ90”ćC£©Ź±»¹Ķ¬Ź±Éś²śĮĖŃĒĀČĖįÄĘ£¬Ę乤ŅÕĮ÷³ĢČēĻĀ£ŗ
¹¤ŅµÉĻÉś²śøßĀČĖį£Ø·Šµć£ŗ90”ćC£©Ź±»¹Ķ¬Ź±Éś²śĮĖŃĒĀČĖįÄĘ£¬Ę乤ŅÕĮ÷³ĢČēĻĀ£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
¹¤ŅµÉĻÉś²śøßĀČĖį£Ø·Šµć£ŗ90oC£©Ź±»¹Ķ¬Ź±Éś²śĮĖŃĒĀČĖįÄĘ£¬Ę乤ŅÕĮ÷³ĢČēĻĀ£ŗ

£Ø1£©ĄäČ“¹żĀĖµÄÄæµÄ ”£
£Ø2£©ĶØČė·“Ó¦Ę÷2µÄSO2×÷ÓĆŹĒ £»·“Ó¦Ę÷2ÖŠ·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ ”£
£Ø3£©Ń»·Ź¹ÓƵÄĪļÖŹŹĒ ”£
£Ø4£©æÉŅŌĶعżÕōĮóĀĖŅŗµÄ·½·ØµĆµ½øßĀČĖįµÄŌŅņæÉÄÜŹĒ ”£
£Ø5£©Ķعżµē½āNaClO3Ė®ČÜŅŗµÄ·½·ØŅ²æÉŅŌÖʱøNaClO4£¬½ų¶ųæÉŅŌÖʱøHClO4£¬Š“³öŃō¼«µÄµē¼«·“Ó¦Ź½ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012½ģ½ĖÕŹ”Ęō¶«ÖŠŃ§øßČżÉĻѧʌµŚ¶ž“ĪŌĀæ¼»ÆѧŹŌ¾ķ ĢāŠĶ£ŗĢīæÕĢā
£Ø12·Ö£©¹¤ŅµÉĻÉś²śøßĀČĖį£Ø·Šµć£ŗ90oC£©Ź±»¹Ķ¬Ź±Éś²śĮĖŃĒĀČĖįÄĘ£¬Ę乤ŅÕĮ÷³ĢČēĻĀ£ŗ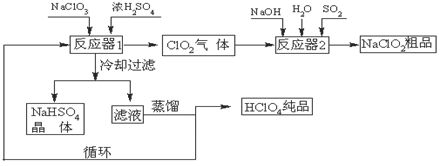
£Ø1£©ĄäČ“¹żĀĖµÄÄæµÄŹĒ½µµĶNaHSO4µÄ ²¢·ÖĄė³öNaHSO4¾§Ģ唣
£Ø2£©·“Ó¦Ę÷2ÖŠ·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ ”£SO2µÄ×÷ÓĆŹĒ×÷ ¼Į”£
£Ø3£©Ń»·Ź¹ÓƵÄĪļÖŹŹĒ ”£
£Ø4£©æÉŅŌĶعżÕōĮóĀĖŅŗµÄ·½·ØµĆµ½øßĀČĖįµÄŌŅņæÉÄÜŹĒ ”£
£Ø5£©¹¤ŅµÉĻÓĆ²¬×÷Ńō¼«”¢Ķ»ņŅų×÷Ņõ¼«µē½āŃĪĖįŅ²æÉÖʵĆøßĀČĖį£¬ŌŚŃō¼«ĒųæɵƵ½20%µÄøßĀČĖį”£Š“³öŃō¼«µÄµē¼«·“Ó¦Ź½£ØĘäÖŠŃĪĖįÓėøßĀČĖįŅŌ»ÆѧŹ½³öĻÖ£© ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013-2014ѧğ¹ć¶«Ź”ÉŲ¹ŲŹŠøßČżµ÷ŃŠ²āŹŌĄķ×Ū»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
¹¤ŅµÉĻÉś²śøßĀČĖį£Ø·Šµć£ŗ90”ćC£©Ź±»¹Ķ¬Ź±Éś²śĮĖŃĒĀČĖįÄĘ£¬Ę乤ŅÕĮ÷³ĢČēĻĀ£ŗ
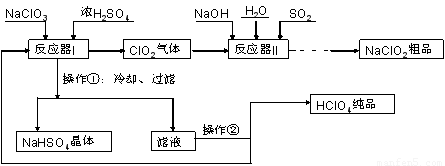
£Ø1£©ŹµŃéŹŅ½ųŠŠ¹żĀĖ²Ł×÷µÄ³£ÓĆ²£Į§ŅĒĘ÷ÓŠ???????????? ”£
£Ø2£©·“Ó¦Ę÷IÖŠµÄĪĀ¶Č×ī¼ŃĪŖ???????? £ØĢīŠņŗÅ£©£»²Ł×÷¢ŚµÄĆū³ĘĪŖ????????? ”£
A. 0”ćC £»????????? B. 20”ćC £»??????? C. 80”ćC £»????????? D. 120”ćC??
£Ø3£©·“Ó¦Ę÷IIÖŠ·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ?????????????? ”£
£Ø4£©¼Óæģ·“Ó¦Ę÷IIÖŠ·“Ó¦ĖŁĀŹµÄ“ėŹ©ÓŠ?????????????? £ØŠ“³öŅ»ÖÖ“ėŹ©¼“æÉ£©µČ”£“Ó·“Ó¦Ę÷IIÖŠ»ńµĆNaClO2?? “ÖĘ·µÄŹµŃé²Ł×÷ŅĄ“ĪŹĒ???????? £ØĢīŠņŗÅ£¬ĻĀĶ¬£©£¬½ųŅ»²½Ģį“æµÄ²Ł×÷Ćū³ĘĪŖ???????? ”£
A£®¹żĀĖ? B£®ÖŲ½į¾§? C£®ÕōĮó? D£®Õō·¢ÅØĖõ? E£®ÕōøÉ×ĘÉÕ? F£®ĄäČ“½į¾§? G£®ŻĶČ”·ÖŅŗ
£Ø5£©ÉĻŹöĮ÷³ĢÖŠæÉŃ»·Ź¹ÓƵÄĪļÖŹĪŖ???????? £¬ø±²śĘ·³żNaClO2”¢NaHSO4Ķā»¹ÓŠ???????? £ØĢī»ÆѧŹ½£©”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com