

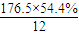 =8��N��H��=
=8��N��H��=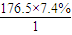 ��13��N��O��=
��13��N��O��= =2��N��Cl��=
=2��N��Cl��=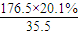 ��1����A�ķ���ʽΪC8H13O2Cl�����ݿ�ͼ��E��G��G��CH3CH��COOH��2����ط�Ӧ����D������������F����֪��GΪCH3CH��CHO��2��EΪCH3CH��CH2OH��2����DΪCH3CH��CH2Cl��CH2OH���ٸ���D��F��H��B��֪��FΪCH3CH��CH2Cl��COOOH��HΪCH2=C��CH3��COOONa��BΪCH2=C��CH3��COOH����A�Ľṹ��ʽΪCH2=C��CH3��COOCH2CH��CH3��CH2Cl������A�ķ���ʽC8H13O2Cl��B�ķ���ʽΪC4H6O2����̼����������ʽ��C-C-C-C��C-C��C��-C����̼̼˫���Ľṹ�У�C-C-C=C��C-C=C-C��C=C��C��2�������������Ļ������У�HCOOCH2CH=CH2��CH3OOC-CH=CH2��CH3COOCH=CH2��HCOOCH=CH-CH3��CH2=C��CH3��-OOCH����5�֣�
��1����A�ķ���ʽΪC8H13O2Cl�����ݿ�ͼ��E��G��G��CH3CH��COOH��2����ط�Ӧ����D������������F����֪��GΪCH3CH��CHO��2��EΪCH3CH��CH2OH��2����DΪCH3CH��CH2Cl��CH2OH���ٸ���D��F��H��B��֪��FΪCH3CH��CH2Cl��COOOH��HΪCH2=C��CH3��COOONa��BΪCH2=C��CH3��COOH����A�Ľṹ��ʽΪCH2=C��CH3��COOCH2CH��CH3��CH2Cl������A�ķ���ʽC8H13O2Cl��B�ķ���ʽΪC4H6O2����̼����������ʽ��C-C-C-C��C-C��C��-C����̼̼˫���Ľṹ�У�C-C-C=C��C-C=C-C��C=C��C��2�������������Ļ������У�HCOOCH2CH=CH2��CH3OOC-CH=CH2��CH3COOCH=CH2��HCOOCH=CH-CH3��CH2=C��CH3��-OOCH����5�֣�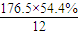 =8��N��H��=
=8��N��H��=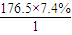 ��13��N��O��=
��13��N��O��=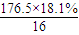 =2��N��Cl��=
=2��N��Cl��=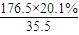 ��1����A�ķ���ʽΪC8H13O2Cl�����ݿ�ͼ��E��G��G��CH3CH��COOH��2����ط�Ӧ����D������������F����֪��GΪCH3CH��CHO��2��EΪCH3CH��CH2OH��2����DΪCH3CH��CH2Cl��CH2OH���ٸ���D��F��H��B��֪��FΪCH3CH��CH2Cl��COOOH��HΪCH2=C��CH3��COOONa��BΪCH2=C��CH3��COOH����A�Ľṹ��ʽΪCH2=C��CH3��COOCH2CH��CH3��CH2Cl������A�ķ���ʽC8H13O2Cl��
��1����A�ķ���ʽΪC8H13O2Cl�����ݿ�ͼ��E��G��G��CH3CH��COOH��2����ط�Ӧ����D������������F����֪��GΪCH3CH��CHO��2��EΪCH3CH��CH2OH��2����DΪCH3CH��CH2Cl��CH2OH���ٸ���D��F��H��B��֪��FΪCH3CH��CH2Cl��COOOH��HΪCH2=C��CH3��COOONa��BΪCH2=C��CH3��COOH����A�Ľṹ��ʽΪCH2=C��CH3��COOCH2CH��CH3��CH2Cl������A�ķ���ʽC8H13O2Cl�� CH2=C��CH3��COOH+ClCH2CH��CH3��CH2OH��
CH2=C��CH3��COOH+ClCH2CH��CH3��CH2OH�� CH2=C��CH3��COOH+ClCH2CH��CH3��CH2OH��
CH2=C��CH3��COOH+ClCH2CH��CH3��CH2OH�� CH3CH��CHO��2+2Cu+2H2O��
CH3CH��CHO��2+2Cu+2H2O�� CH3CH��CHO��2+2Cu+2H2O��
CH3CH��CHO��2+2Cu+2H2O��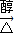 CH2=C��CH3��COOONa+NaCl+2H2O��
CH2=C��CH3��COOONa+NaCl+2H2O��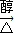 CH2=C��CH3��COOONa+NaCl+2H2O��
CH2=C��CH3��COOONa+NaCl+2H2O��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

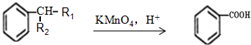





 ��
��
 ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ϡ���� |
| �� |
| ϡ���� |
| �� |
| �� |
| �� |
| �� |
| �� |
| �� |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�갲��ʡ����ɽ�б�ҵ���һ�ν�ѧ����������ۻ�ѧ�Ծ��������棩 ���ͣ������
���ܼ���һ�����Ӳ��ϵ������Ի�ʹ����Һ�������Ӽ�����������ʳƷ���������ҵ��DBP�����ܼ���һ�֣���������·�ߺϳɡ�
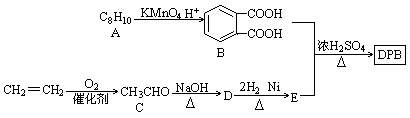
��֪������Ϣ��
�� 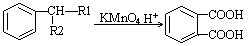
�� 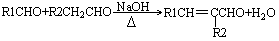
����R1��һR2��ʾ��ԭ�ӻ�������
��1��A�Ľṹ��ʽ ��D��E�ķ�Ӧ���� ��
��2��B�������� ��D�к��еĹ����������� ��
��3����B��E�ϳ�DBP�Ļ�ѧ����ʽ ��
��4��д��ͬʱ��������������B������ͬ���칹��ṹ��ʽ
���ܺ�NaHCO3��Һ��Ӧ����CO2 ����ʹFeC13��Һ������ɫ��Ӧ
���ܷ���������Ӧ �ܱ����Ϻ�̼���Ŵ��ڶ�λ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com