
| ʵ����� | ��һ�� | �ڶ��� | ������ |
| ����NaOH��Һ���/mL | 26.02 | 25.35 | 25.30 |
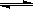 H++OH-��KW=10-14�� CH3COOH
H++OH-��KW=10-14�� CH3COOH 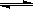 H++ CH3COO����Ka=1.8��10-5
H++ CH3COO����Ka=1.8��10-5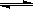 CH3COOH+OH-������3�֣�
CH3COOH+OH-������3�֣�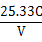
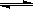 H++ CH3COO��������������ƹ���ʱ��CH3COO��Ũ��������ƽ�������ƶ������½������C��H+����С��C��CH3COOH����������C��H+����C��CH3COOH���ı�ֵ��С��
H++ CH3COO��������������ƹ���ʱ��CH3COO��Ũ��������ƽ�������ƶ������½������C��H+����С��C��CH3COOH����������C��H+����C��CH3COOH���ı�ֵ��С��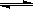 CH3COOH+OH-��ˮ�������ȵģ����¶�������ƽ�������ƶ�������C(OH��)����
CH3COOH+OH-��ˮ�������ȵģ����¶�������ƽ�������ƶ�������C(OH��)����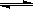 CH3COOH+OH-��1mol��L-1��������Һ�൱����0��5mol��L-1�����ϼ�������ƣ�ˮ��ƽ�������ƶ���C(OH��) ���ߣ�����m<n,�����ֻ�ѧƽ��ֻ�����ƴ����Ƽ����Ӱ�죬�����ܵ���������ˮ��ȵĶ��壬����a>b��
CH3COOH+OH-��1mol��L-1��������Һ�൱����0��5mol��L-1�����ϼ�������ƣ�ˮ��ƽ�������ƶ���C(OH��) ���ߣ�����m<n,�����ֻ�ѧƽ��ֻ�����ƴ����Ƽ����Ӱ�죬�����ܵ���������ˮ��ȵĶ��壬����a>b��

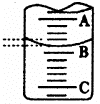
 Сѧ���AB��ϵ�д�
Сѧ���AB��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ���ֽ�������ʵ
���ֽ�������ʵ �飺
�飺 Һ�п϶����ڵ�������__________ _______���϶������ڵ�������________ _____��
Һ�п϶����ڵ�������__________ _______���϶������ڵ�������________ _____�� ����д�����з�����Ӧ�����ӷ�Ӧ����ʽ
����д�����з�����Ӧ�����ӷ�Ӧ����ʽ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��������ˮ�� Cl����NO3����Na����SO32�� | B��ǿ���Ե���Һ�� C6H5O����K����SO42����Br- |
| C��Na2S��Һ�� SO42����K����Cl����Cu2�� | D��ǿ���Ե���Һ�� NO3����I����Na����AlO2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��K+��SiO32����NO3�� | B��NH4+��Al3+��SO42һ |
| C��Na+��OHһ��SO42һ | D��Na+��CH3COһ��ClOһ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��CO32��? | B��NH4+ | C��SO42�� | D��NO3�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Fe3����H����Cl����SCN�� | B��Fe2����SO42����H����NO3�� |
| C��Ba2����HCO3����Cl����Na�� | D��K����NH4����SO42����OH�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����������Һ��ˮϡ�ͣ�����̶�����H+Ũ������ |
| B��ͬ���ͬpH������ʹ�����������п��Ӧ������ķ�Ӧ���ʿ죬����H2�����ʵ����� |
C��NaHCO3��Һ�������Ե�ԭ��HCO3-+H2O CO32-+H3O+ CO32-+H3O+ |
| D��Na2CO3��Һ�����㣺2c(Na+)=c(CO32-)+ c(HCO3-)+ c(H2CO3) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ڢۢ� | B���٢ۢ� | C���ڢܢ� | D���٢ڢ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com