
��14�֣�AΪ���ʣ�B��C��D��EΪ��A������ͬԪ�صĻ��������֮��������ת����ϵ��
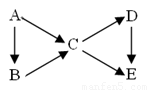
��1������ͼ��B��C��Ϊ�����D��E��Ϊ�Σ���A������__________��
��Na ��N2 ��C ��S
��2�����������ʵ���ɫ��Ӧ��Ϊ��ɫ������C��D��E��ˮ��Һ
���Լ��ԣ��ҵ�Ũ��ʱ����C��D��E��B��������������B�к��еĻ�ѧ������Ϊ__________________��
Aת����C�����ӷ���ʽΪ�� ________________________________��
Dת����E�����ӷ���ʽΪ_______________________________________��
��3����������B��C��D��Ϊ���壬��B������ʹʪ��ĺ�ɫʯ����ֽ����
���ڹ�ҵ������B����ʱΪ�˼ӿ췴Ӧ����Ӧѡ���������______________����������߷�Ӧ���ת���ʵ�������___________��
��C��D������β���е��к��ɷ֣���NaOH��Һ���տ�������Ⱦ����Ӧ�Ļ�ѧ����ʽΪ__________________________________��
 ��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
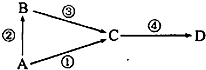 A��B��C��D��Ϊ��ѧ�������ʣ�����AΪ���ʣ�B��C��DΪ��������Ҵ�������ת����ϵ���Իش��������⣺
A��B��C��D��Ϊ��ѧ�������ʣ�����AΪ���ʣ�B��C��DΪ��������Ҵ�������ת����ϵ���Իش��������⣺
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��14�֣�AΪ���ʣ�B��C��D��EΪ��A������ͬԪ�صĻ��������֮��������ת����ϵ��
��1������ͼ��B��C��Ϊ�����D��E��Ϊ�Σ���A������__________��
��Na ��N2 ��C ��S
��2�����������ʵ���ɫ��Ӧ��Ϊ��ɫ������C��D��E��ˮ��Һ
���Լ��ԣ��ҵ�Ũ��ʱ����C��D��E��B��������������B�к��еĻ�ѧ������Ϊ__________________��
Aת����C�����ӷ���ʽΪ��________________________________��
Dת����E�����ӷ���ʽΪ_______________________________________��
��3����������B��C��D��Ϊ���壬��B������ʹʪ��ĺ�ɫʯ����ֽ����
���ڹ�ҵ������B����ʱΪ�˼ӿ췴Ӧ����Ӧѡ���������______________����������߷�Ӧ���ת���ʵ�������___________��
��C��D������β���е��к��ɷ֣���NaOH��Һ���տ�������Ⱦ����Ӧ�Ļ�ѧ����ʽΪ__________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ʡ������ѧ�ڵڶ����¿���ѧ�Ծ� ���ͣ������
(9��)AΪ���ʣ�B��C��D��EΪ��A������ͬԪ�صĻ��������֮��������ת����ϵ��

(1)���������ʵ���ɫ��Ӧ��Ϊ��ɫ������C��D��E��ˮ��Һ���Լ��ԣ��ҵ�Ũ��ʱ����C��D��E��B������������
��B�ĵ���ʽ__________��C�к��еĻ�ѧ������Ϊ__________ (����Ӽ����������Լ����Ǽ��Լ�������
��Aת����C�����ӷ���ʽΪ: ____________________
(2)��������A��B��C��D��Ϊ����,��DΪ����ɫ���塣
��I)���ʵĻ�ѧʽ��__________.д��B��C�Ļ�ѧ����ʽΪ____________________
�ڽ�B��CO2����ͨ�뱥��ʳ��ˮ����̼�����ƾ�����������Ӧ�����ӷ���ʽ�ǣ�__________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ʡ����11��������ѧ�Ծ� ���ͣ������
��8�֣�
A��B��C��D��Ϊ��ѧ�������ʣ�����AΪ���ʣ�B��C��DΪ��������Ҵ�������ת����ϵ��
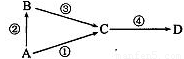
�Իش��������⣺
��1����B��C�����D���
a����AΪ�ǽ�������ʱ����Ӧ�۵Ļ�ѧ����ʽΪ ��
b����AΪ��������ʱ����Ӧ��������1mol����ת�Ƶ�����Ϊ mol��
��2����AΪ����ɫ�ǽ������ʣ�BΪ��̬�⻯�CΪ��̬����� B��C��Ӧ���������뻹ԭ����������Ϊ ��
��3����AΪ�������ʣ�BΪ������ҷ�Ӧ�٢۾�����ǿ����Һ�����ķ�Ӧ����Ӧ�ٵ����ӷ���ʽΪ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com