
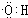 ��DΪCH3COOH�������Ȼ���
��DΪCH3COOH�������Ȼ���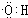 ���Ȼ���
���Ȼ��� 2CH3CHO+2H2O��
2CH3CHO+2H2O��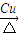 2CH3CHO+2H2O��
2CH3CHO+2H2O��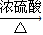 CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O��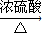 CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O��

 ͬ��������ϰϵ�д�
ͬ��������ϰϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�



| Cu |
| �� |
| Cu |
| �� |
| Ũ���� |
| �� |
| Ũ���� |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
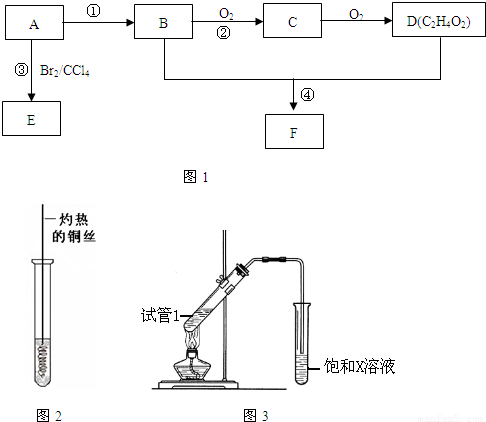
ij��̬��A�ڱ�״���µ��ܶ�Ϊ1.25g/L�������������������һ�����ҵ�ʯ�ͻ�����չˮƽ��B��D���������г������л��D�ܸ�̼�����Ʒ�Ӧ��F����ζ������֮���ת����ϵ����ͼ��ʾ��

��1��A�ĽṹʽΪ ��B�й����ŵĵ���ʽΪ ��
D�й����ŵ�����Ϊ ��
��2����Ӧ�ٵķ�Ӧ������ ��
��Ӧ�۵Ļ�ѧ����ʽΪ ��
��3����Ӧ����Cu�������������½��У���ʵ��IJ����ǽ�������ͭ˿���ھƾ����ϼ��ȣ���ͭ˿��Ϊ��ɫʱ��Ѹ�ٽ�����뵽װ��B���Թ��У���ͼ��ʾ�����ظ�����2��3�Ρ��÷�Ӧ�Ļ�ѧ����ʽΪ ��
��4��D��̼��������Һ��Ӧ�����ӷ���ʽΪ ��
��5��B��D��Ũ�����������ʵ�ַ�Ӧ�ܣ�ʵ��װ������ͼ��ʾ��

�Թ�1��װ��ҩƷ����ȡ�ͼ��X�Ļ�ѧʽΪ ��

�������� ��
�Թ�1��Ӧ�Ļ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�걱�����и߶���ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
ij��̬��A�ڱ�״���µ��ܶ�Ϊ1.25g/L�������������������һ�����ҵ�ʯ�ͻ�����չˮƽ��B��D���������г������л��D�ܸ�̼�����Ʒ�Ӧ��F����ζ������֮���ת����ϵ����ͼ��ʾ��

 ��1��A�ĽṹʽΪ ��B�й����ŵĵ���ʽΪ ��
��1��A�ĽṹʽΪ ��B�й����ŵĵ���ʽΪ �� D�й����ŵ�����Ϊ ��
D�й����ŵ�����Ϊ �� ��2����Ӧ�ٵķ�Ӧ������ ��
��2����Ӧ�ٵķ�Ӧ������ ��
��Ӧ�۵Ļ�ѧ����ʽΪ ��
��
 ��3����Ӧ����Cu�������������½��У���ʵ��IJ����ǽ�������ͭ˿���ھƾ����ϼ��ȣ���ͭ˿��Ϊ��ɫʱ��Ѹ�ٽ�����뵽װ��B���Թ��У���ͼ��ʾ�����ظ�����2��3�Ρ��÷�Ӧ�Ļ�ѧ����ʽΪ ��
��3����Ӧ����Cu�������������½��У���ʵ��IJ����ǽ�������ͭ˿���ھƾ����ϼ��ȣ���ͭ˿��Ϊ��ɫʱ��Ѹ�ٽ�����뵽װ��B���Թ��У���ͼ��ʾ�����ظ�����2��3�Ρ��÷�Ӧ�Ļ�ѧ����ʽΪ �� ��4��D��̼��������Һ��Ӧ�����ӷ���ʽΪ ��
��4��D��̼��������Һ��Ӧ�����ӷ���ʽΪ �� ��5��B��D��Ũ�����������ʵ�ַ�Ӧ�ܣ�ʵ��װ������ͼ��ʾ��
��5��B��D��Ũ�����������ʵ�ַ�Ӧ�ܣ�ʵ��װ������ͼ��ʾ��
 �Թ�1��װ��ҩƷ����ȡ�ͼ��X�Ļ�ѧʽΪ ��
�Թ�1��װ��ҩƷ����ȡ�ͼ��X�Ļ�ѧʽΪ ��
 �������� ��
�������� �� �Թ�1��Ӧ�Ļ�ѧ����ʽΪ ��
�Թ�1��Ӧ�Ļ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��߿���ѧ���ָ�ϰ�Ų� ר��15�л���ѧ����ѡ��5��ϰ���������棩 ���ͣ������
ij��̬��A�ڱ�״���µ��ܶ�Ϊ1.25 g/L�������������������һ�����ҵ�ʯ�ͻ�����չˮƽ��B��D���������г������л�����D�ܸ�̼�����Ʒ�Ӧ��F����ζ������֮���ת����ϵ������ʾ��

��1��A�ĽṹʽΪ����������B�й����ŵĵ���ʽΪ����������
��2����Ӧ���ķ�Ӧ������������������Ӧ���Ļ�ѧ����ʽΪ��������������������������
��3����Ӧ����Cu�������������½�������ʵ��IJ����ǽ�������ͭ˿�����ƾ����ϼ��ȣ���ͭ˿��Ϊ��ɫʱ��Ѹ�ٽ�����뵽װ��B���Թ��У���ͼ��ʾ�����ظ�����2��3�Ρ��÷�Ӧ�Ļ�ѧ����ʽΪ______________________��

��4��D��̼��������Һ��Ӧ�����ӷ���ʽΪ��������������������������
��5��B��D��Ũ����������·�����Ӧ�ܣ�ʵ��װ����ͼ��ʾ��

�Թ���װ��ҩƷ�����ȡ�ͼ��X�Ļ�ѧʽΪ����������������____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�걱�����и߶���ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
ij��̬��A�ڱ�״���µ��ܶ�Ϊ1.25g/L�������������������һ�����ҵ�ʯ�ͻ�����չˮƽ��B��D���������г������л��D�ܸ�̼�����Ʒ�Ӧ��F����ζ������֮���ת����ϵ����ͼ��ʾ��


 ��1��A�ĽṹʽΪ ��B�й����ŵĵ���ʽΪ
��
��1��A�ĽṹʽΪ ��B�й����ŵĵ���ʽΪ
��
 D�й����ŵ�����Ϊ
��
D�й����ŵ�����Ϊ
��
 ��2����Ӧ�ٵķ�Ӧ������ ��
��2����Ӧ�ٵķ�Ӧ������ ��
��Ӧ�۵Ļ�ѧ����ʽΪ ��
��

 ��3����Ӧ����Cu�������������½��У���ʵ��IJ����ǽ�������ͭ˿���ھƾ����ϼ��ȣ���ͭ˿��Ϊ��ɫʱ��Ѹ�ٽ�����뵽װ��B���Թ��У���ͼ��ʾ�����ظ�����2��3�Ρ��÷�Ӧ�Ļ�ѧ����ʽΪ
��
��3����Ӧ����Cu�������������½��У���ʵ��IJ����ǽ�������ͭ˿���ھƾ����ϼ��ȣ���ͭ˿��Ϊ��ɫʱ��Ѹ�ٽ�����뵽װ��B���Թ��У���ͼ��ʾ�����ظ�����2��3�Ρ��÷�Ӧ�Ļ�ѧ����ʽΪ
��
 ��4��D��̼��������Һ��Ӧ�����ӷ���ʽΪ
��
��4��D��̼��������Һ��Ӧ�����ӷ���ʽΪ
��
 ��5��B��D��Ũ�����������ʵ�ַ�Ӧ�ܣ�ʵ��װ������ͼ��ʾ��
��5��B��D��Ũ�����������ʵ�ַ�Ӧ�ܣ�ʵ��װ������ͼ��ʾ��

 �Թ�1��װ��ҩƷ����ȡ�ͼ��X�Ļ�ѧʽΪ
��
�Թ�1��װ��ҩƷ����ȡ�ͼ��X�Ļ�ѧʽΪ
��

 ��������
��
��������
��
 �Թ�1��Ӧ�Ļ�ѧ����ʽΪ
��
�Թ�1��Ӧ�Ļ�ѧ����ʽΪ
��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com