
| ¢ńA | ||||||||
| 1 | ¢Ł | ¢ņA | ¢óA | ¢ōA | ¢õA | ¢öA | ¢÷A | |
| 2 | ¢Ś | ¢Ū | ¢Ü | ¢Ż | ||||
| 3 | ¢Ž | ¢ß | ¢ą | ¢į | ||||
| 4 | =10 ¢ā | |||||||
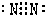 £¬¹Ź“š°øĪŖ£ŗ
£¬¹Ź“š°øĪŖ£ŗ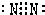 £»
£»

 ŠĀæĪ±źæģĄÖĢįÓÅŹī¼Ł×÷ŅµÉĀĪ÷ĀĆÓĪ³ö°ęÉēĻµĮŠ“š°ø
ŠĀæĪ±źæģĄÖĢįÓÅŹī¼Ł×÷ŅµÉĀĪ÷ĀĆÓĪ³ö°ęÉēĻµĮŠ“š°ø Źī¼ŁĻĪ½ÓÅąÓŽĢ²ÄÕć½¹¤ÉĢ“óѧ³ö°ęÉēĻµĮŠ“š°ø
Źī¼ŁĻĪ½ÓÅąÓŽĢ²ÄÕć½¹¤ÉĢ“óѧ³ö°ęÉēĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| ¢ńA | 0 | |||||||
| 1 | ¢Ł | ¢ņA | ¢óA | ¢ōA | ¢õA | ¢öA | ¢÷A | |
| 2 | ¢Ś | ¢Ü | ¢Ż | |||||
| 3 | ¢Ž | ¢ß | ¢Ū | ¢ą | ¢į | |||
| 4 | ¢ā | |||||||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| ¢ńA | 0 | |||||||
| 1 | ¢Ł | ¢ņA | ¢óA | ¢ōA | ¢õA | ¢öA | ¢÷A | |
| 2 | ¢Ś | ¢Ū | ¢Ü | ¢Ż | ||||
| 3 | ¢Ž | ¢ß | ¢ą | ¢į | ||||
| 4 | =10 ¢ā | |||||||
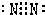
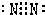
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| ×å ÖÜĘŚ |
IA | IIA | IIIA | IVA | VA | VIA | VIIA | 0 |
| ¶ž | ¢Ż | ¢Ž | ¢ß | ¢į | ||||
| Čż | ¢Ł | ¢Ū | ¢Ü | ¢ą | ¢ā | |||
| ĖÄ | ¢Ś |  |
 Į½ŌŖĖŲŗĖµēŗÉÖ®²īŹĒ
Į½ŌŖĖŲŗĖµēŗÉÖ®²īŹĒ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| ×åÖÜĘŚ | ¢ńA | ¢ņA | ¢óA | ¢ōA | ¢õA | ¢öA | ¢÷A |
0 |
| 2 | ¢Ž | ¢ß | ||||||
| 3 | ¢Ł | ¢Ū | ¢Ż | ¢ą | ||||
| 4 | ¢Ś | ¢Ü |


²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com