
����Ŀ����ͭ����Ҫ�ɷ���Cu2S����ұ��ͭ����Ҫԭ�ϣ���ͭ����Ʒ�Ĵ��ȿ��������Ը��������Һ�ζ����ⶨ���÷�Ӧ�ɱ�ʾΪCu2S��![]() ��H����Cu2����
��H����Cu2����![]() ��Mn2����H2O��δ��ƽ��������˵���в���ȷ����
��Mn2����H2O��δ��ƽ��������˵���в���ȷ����
A. Cu2SΪ��ԭ���������� ![]() Ϊ����������ԭ
Ϊ����������ԭ
B. �������ͻ�ԭ�����ʵ���֮��Ϊ2��1
C. ��Ӧ��ÿ����1 mol Cu2S��ת��8 mol����
D. �ζ�ʱ���Բ���������ָʾ��
���𰸡�C
����������ƽ���ӷ���ʽΪCu2S��2MnO4-��8H+=2Cu2+��SO42-��2Mn2+��4H2O
A�Cu2S��ͭԪ�ػ��ϼ��ɣ�1����2�ۣ�����������Ԫ�ػ��ϼ���-2����6�ۣ�������������Cu2S����ԭ����MnO4-����Ԫ�ػ��ϼۣ�7����2�ۣ�����ԭ������MnO4-������������A��ȷ��B����ݷ���ʽ�������������������ͻ�ԭ�������ʵ���֮��2:1����B��ȷ��C���Ӧ��ÿ����1molCu2S��ת��10mol���ӣ���C������D���ΪMnO4-����������ɫ�����Եζ�ʱ���ü�����ָʾ������D��ȷ��


 ��ٽ������½������������ϵ�д�
��ٽ������½������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ԫ��R����̬�⻯����H2R��RΪ-2�ۣ�������������������Ӧ��ˮ����Ļ�ѧʽ���� ��
A. HRO3 B. HRO4 C. H2RO3 D. H2RO4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ��һ����ʵ��Ϊ������ѧ�ƣ���ѧʵ���ǻ�ѧѧϰ����Ҫ���ݡ�
��1��������ʵ���ҳ��õIJ���������

������f��j�����Ʒֱ�Ϊ_____��______��
���ڷ�Һ�����У������õ���������_________(����ĸ,��ͬ)��
��������Ӧ�����ҿ�ֱ�Ӽ��ȵ�������_________��
�ܹ���ʱ,��Ҫ�õ��IJ���������_________
��2������ʵ�������������,��ȷ����______(����ĸ)��
A.����ˮ��ʪ��pH��ֽ����ϡ�����У��ⶨ��Һ��pH
B.��ϡ����ϴ��ʢ�Ź�ʯ��ˮ���Լ�ƿ
C.ϡ��Ũ����ʱ���������ձ��м���һ�������Ũ���ᣬ���ڽ�������������ˮ
D.����һ��������ͬ����ֽ����ƽ���������ϣ���NaOH�����������ֽ�ϳ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����80 ��ʱ����0.40 mol��N2O4�������2 L�Ѿ���յĹ̶��ݻ����ܱ������У��������·�Ӧ��N2O4![]() 2NO2 ��H��0����һ��ʱ��Ը������ڵ����ʽ��з������õ��������ݣ�
2NO2 ��H��0����һ��ʱ��Ը������ڵ����ʽ��з������õ��������ݣ�
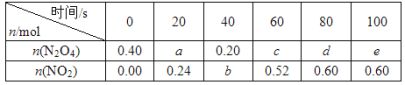
��1������20~40 s����N2O4��ʾ��ƽ����Ӧ����Ϊ_________mol/( L s)��
��2��������80��ʱ�÷�Ӧ��ƽ�ⳣ��K=__________(��ע����λ)��
��3����Ӧ������100 s��Ӧ�������¶Ƚ��ͣ�����������ɫ______(���dz����������䡱)��
��4��Ҫ����÷�Ӧ��Kֵ���ɲ�ȡ�Ĵ�ʩ��_________(�����)��
A������N2O4����ʼŨ��
B������������ͨ��NO2
C��ʹ�ø�Ч����
D�������¶�
��5����ͼ��80��ʱ������N2O4���ʵ����ı仯���ߣ����ڸ�ͼ�в������÷�Ӧ��60��ʱN2O4���ʵ����ı仯���ߡ�
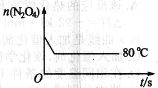
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͭ�����Ҫ�ɷ���Cu2O����ͭ�����Ҫ�ɷ���Cu2S������ͭ�����ͭ���ϼ��������·�Ӧ��Cu2S��2Cu2O![]() 6Cu��SO2�������й��ڸ÷�Ӧ��˵���У���ȷ����
6Cu��SO2�������й��ڸ÷�Ӧ��˵���У���ȷ����
A. ÿ����22.4L SO2����Ӧ��ת��6 mol����
B. Cu2S�ڷ�Ӧ�м��������������ǻ�ԭ��
C. Cu���������������ǻ�ԭ����
D. ÿ����19.2 g Cu����Ӧ��ת��0.6 mol����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���Ժ�Ǧ����(��Ҫ��Pb��PbO��PbO2��PbSO4)��ϡH2SO4Ϊԭ���Ʊ��ߴ�Pb��PbO�ȣ�ʵ��Ǧ���������á�����Ҫ�������£�

(1)�����ܡ�ʱ����Fe2+���£�Pb��PbO2��Ӧ����PbSO4������1mol PbSO4��ת�Ƶ��ӵ����ʵ�����________mol��Fe2+�����̿ɱ�ʾΪ��
��2Fe2++PbO2+4H++SO42-=2Fe3++PbSO4+2H2O
��______________________��(�����ӷ���ʽ��ʾ��Ӧ��)
(2)д��������̷�����Ҫ��Ӧ�Ļ�ѧ����ʽ��_______________________________��
(3)��֪����PbO�ܽ���NaOH��Һ�У�����ƽ�⣺PbO(s)+NaOH(aq)==NaHPbO2(aq)�����ܽ����������ͼ��ʾ��

�ڴ�ƷPbO���������ʲ�����NaOH��Һ�����������Ϣ������ɴ�ƷPbO�õ��ߴ�PbO�IJ���������ƷPbO�ܽ���һ����______(�35������10����)��NaOH��Һ�У�������110�棬����ܽ��_________(����ȹ��ˡ�������Ũ����)������Һ��ȴ�ᾧ�����ˡ�ϴ�Ӳ�����õ��ߴ�PbO���塣
(4)��PbO��Ʒ�ܽ���HC1��NaC1�Ļ����Һ�У��õ���Na2PbC14�ĵ��Һ�����Na2PbC14��Һ������Pb������ͼ��ʾ��

�������ĵ缫��Ӧʽ��__________________________��
�ڵ��һ��ʱ���Na2PbC14Ũ�ȼ����½���Ϊ�˻ָ���Ũ����ʵ�����ʵ�ѭ�����ã���������ȡ�ķ�����________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������п����п������Ҫ�Ļ�������ԭ�ϡ�
��1��ZnO �� Al2O3 �Ļ�ѧ�������ƣ�ZnO �� NaOH ��Һ��ת����[Zn(OH)4]2�����ӷ���ʽΪ_____________��
��2������п�õ�������п�к���Ǧ��ͭ�����ʣ��ᴿ�������£�
![]()
![]()
����ͼ�еġ������Ϊ________���ѧʽ����

��ij�¶�ʱ���ڷ�Ӧ��ķ�Ӧ¯�У���ʼʱ c(CO)Ϊ 0.3 molL1����Ӧ������ CO2 ��������� ��(CO2)��ͼ��ʾ����Ӧ���ƽ�ⳣ�� K��_____��
�����д�ʩ��������߷�Ӧ���� ZnO ת���ʵ���________��
a������ ZnO ��Ͷ���� b���ʵ���ѹ c����п������ʱ����
�ܷ�Ӧ���У�ÿת�� 1mol ���ӣ���Ӧ���� 174 kJ���� H2��_____________��
��3���ⶨ����п��Ʒ���ȣ���ȡ 0.5000g ��Ʒ�����ܺ����� 250 mL ����ƿ�У�ҡ�ȡ���ȡ 25.00 mL ����Һ���� 0.04000 molL1 �� EDTA��Na2H2Y����Һ�ζ����е� Zn2+����Ӧ����ʽΪ Zn2+��H2Y2��ZnY2��2H+�����ʲ���Ӧ����ƽ�еζ����Σ�ƽ������ EDTA ��Һ 15.12mL��
�����ζ���δ�� EDTA ��Һ��ϴ���ⶨ�����___���ƫ�ߡ�����ƫ�͡����䡱����
����Ʒ����Ϊ��________________���г�����ʽ���ɣ���
��4���ʵ�ӫ�����е���ɫӫ��ۺ��� ZnS�������� 0.05mol ZnS ��ӫ������� 500mL�����У���ȫ�ܽ����Һ�� c(S2)��__________ molL1������֪��Ksp(ZnS)��2.5��1023��������Һ����ı仯��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪A��BΪ���嵥�ʣ�����һ��Ϊ����ɫ�����߷�Ӧ��������C��DΪ�����������ʣ�E��ҺΪdz��ɫ������֮��������ת����ϵ��

��1��д��C����Һ��D��Ӧ�����ӷ���ʽ��___________��
��2��д��Aͨ��E��Һ����F�����ӷ���ʽ��___________��
��3��E��Һ�к���F����ȥFӦ�����Լ�________����Ӧ�����ӷ�Ӧ����ʽΪ��___________��
��4������F�е�������ʱ���ɼ����Լ�X����Һ�������ǣ�______________��X�Լ��Ļ�ѧʽ�ǣ�___________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ���⣺��ͼ��ij�����Լ�ƿ��ǩ�ϵ����ݡ�

��1������������ʵ���Ũ����____________mol/L��
��2��ij��ѧ��ȤС������������ʵ�ʵ��̽��ʱ������490 mL 4.6 mol/L��ϡ���ᣬ��Ҫ��ȡ����������Ϊ_____________mL��
��3�����⣨2����Ҫ��������Һʱ����ͼ�п϶�����Ҫ�õ���ʵ��������________(���������)������IJ��������У�����Ͳ���ձ����������ͽ�ͷ�ι�֮�⣬����Ҫ_______________(����������)��

��4�����������ƹ���ʾ��ͼ�У��д������(��д���) __________________��

��5��������4.6 mol/L��ϡ����Ĺ����У��������������������Һ���ʵ���Ũ��ƫ�͵���_____��
A��δ����ȴ���Ƚ���Һע������ƿ��
B������ƿϴ�Ӻ�δ�����ﴦ��
C������ʱ���ӹ۲�Һ��
D��ҡ�Ⱥ���Һ����ڿ̶�������ˮ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com