
���ס�������������ͬ�������壬�ڿ�����ȼ�յõ����������������ʱ����P4O6����������ʱ����P4O10��
��1����֪298 Kʱ���ס�������ȫȼ�յ��Ȼ�ѧ����ʽ�ֱ�ΪP4��s�����ף���5O2��g��=P4O10��s����H1����2 983.2 kJ��mol��1��P��s�����ף��� O2��g��=
O2��g��=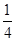 P4O10��s����H2����738.5 kJ��mol��1������¶��°���ת��Ϊ�����Ȼ�ѧ����ʽΪ________________��
P4O10��s����H2����738.5 kJ��mol��1������¶��°���ת��Ϊ�����Ȼ�ѧ����ʽΪ________________��
��2����֪298 Kʱ���ײ���ȫȼ�յ��Ȼ�ѧ����ʽΪP4��s�����ף���3O2��g��=P4O6��s����H����1 638 kJ��mol��1����ij�ܱ������м���62 g����50.4 L��������״��������������ʹ֮ǡ����ȫ��Ӧ�������õ���P4O10��P4O6�����ʵ���֮��Ϊ________����Ӧ�����зų�������Ϊ________��
��3����֪����PCl3�ķ��ӽṹ��ͼ��ʾ�����ṩ���»�ѧ���ļ��ܣ�kJ��mol��1����P��P 198��Cl��Cl 243��P��Cl 331��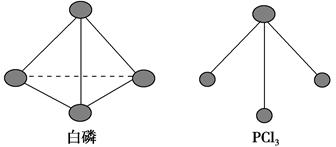
��ӦP4��s�����ף���6Cl2��g��=4PCl3��s���ķ�Ӧ�Ȧ�H��________��
 ���ƽ̸�������ѡ����ĩ���100��ϵ�д�
���ƽ̸�������ѡ����ĩ���100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������Ϊһ������Դ�ڻ�ѧ����Ӧ�ù㷺����ش��������⣺
(1)��¯ұ�������У������ڴ���Ӧ���в���ˮú��(CO��H2)��ԭ���������йط�ӦΪ��CH4(g)��CO2(g)=2CO(g)��2H2(g)����H��260 kJ��mol��1
��֪��2CO(g)��O2(g)=2CO2(g)����H����566 kJ��mol��1
��CH4��O2��Ӧ����CO��H2���Ȼ�ѧ����ʽΪ_____________________��
(2)����ͼ��ʾ��װ�â�Ϊ����ȼ�ϵ��(�������ҺΪKOH��Һ)��ͨ��װ�â�ʵ�������϶�ͭ��
��a��Ӧͨ��________(�CH4����O2��)��b���缫�Ϸ����ĵ缫��Ӧʽ��________��
�ڵ�ƽ�����װ�â�����Һ��pH________(��д�������С�����䡱����ͬ)��װ�â���Cu2�������ʵ���Ũ��________��
�۵�ƽ�����װ�â���Һ�е������ӳ���OH���������________(����ˮ��)��
���ڴ˹���������ȫ��Ӧ��װ�â������������仯12.8 g����װ�â������������ļ���________L(��״����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ú������Һ�����ִ���Դ��ҵ���ص㿼�ǵ���Դ�ۺ����÷������������������Ϊ��ú����ˮú��������ǰ�Ƚ����е�Һ������Ϊ��ú����CH3OH��
��1����֪��CO2(g)��3H2(g)=CH3OH(g)��H2O(g)����H1
2CO(g)��O2(g)=2CO2(g)����H2
2H2(g)��O2(g)=2H2O(g)����H3
��ӦCO(g)��2H2(g)=CH3OH(g)�Ħ�H��______��
��2����ͼ�Ǹ÷�Ӧ�ڲ�ͬ�¶���CO��ת������ʱ��仯�����ߡ�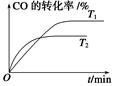
��T1��T2�¶��µ�ƽ�ⳣ����С��ϵ��K1________K2(�����������������)��
����CO�ϳɼ״�ʱ��CO��250 �桢300 �桢350 ���´ﵽƽ��ʱת������ѹǿ�Ĺ�ϵ��������ͼ��ʾ��������c����ʾ���¶�Ϊ________ �档ʵ����������������250 �桢1.3��104 kPa���ң�ѡ���ѹǿ��������____________��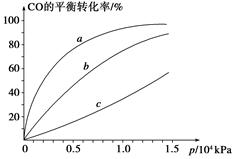
�������йظ÷�Ӧ��˵����ȷ����________(�����)��
A�����¡����������£��������ڵ�ѹǿ�������仯������淴Ӧ�ﵽƽ��
B��һ�������£�H2������������CO���������ʵ�2��ʱ�����淴Ӧ�ﵽƽ��
C��ʹ�ú��ʵĴ��������̴ﵽƽ���ʱ�䲢���CH3OH�IJ���
D��ij�¶��£���2 mol CO��6 mol H2����2 L�ܱ������У���ַ�Ӧ���ﵽƽ����c(CO)��0.2 mol��L��1����CO��ת����Ϊ80%
��3��һ���¶��£���2 L�̶�������ܱ������м���1 mol CH3OH(g)��������Ӧ��CH3OH(g)??CO(g)��2H2(g)��H2�����ʵ�����ʱ��仯��������ͼ��ʾ��
0��2 min�ڵ�ƽ����Ӧ����v(CH3OH)��__________�����¶��£���ӦCO(g)��2H2(g)??CH3OH(g)��ƽ�ⳣ��K��__________����ͬ�¶��£�����ʼʱ����CH3OH(g)�����ʵ�����ԭ����2������__________(�����)��ԭ����2����
A��ƽ�ⳣ�� B��CH3OH��ƽ��Ũ��
C���ﵽƽ���ʱ�� D��ƽ��ʱ������ܶ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����������������йط�Ӧ���ʱ䣺
��1����֪��Ti(s)��2Cl2(g)=TiCl4(l)��H����804��2 kJ��mol��1
2Na(s)��Cl2(g)=2NaCl(s)����H����882��0 kJ��mol��1
Na(s)=Na(l)����H����2��6 kJ��mol��1
��ӦTiCl4(l)��4Na(l)=Ti(s)��4NaCl(s)�Ħ�H��________ kJ��mol��1��
��2����֪���з�Ӧ��ֵ��
| ��Ӧ��� | ��ѧ��Ӧ | ��Ӧ�� |
| �� | Fe2O3(s)��3CO(g)= 2Fe(s)��3CO2(g) | ��H1����26��7 kJ��mol��1 |
| �� | 3Fe2O3(s)��CO(g)=2Fe3O4(s)��CO2(g) | ��H2����50��8 kJ��mol�� |
| �� | Fe3O4(s)��CO(g)=3FeO(s)��CO2(g) | ��H3����36��5 kJ��mol��1 |
| �� | FeO(s)��CO(g)=Fe(s)��CO2(g) | ��H4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1����������������ʱ������N2��O2��Ӧ���������仯ʾ��ͼ���£�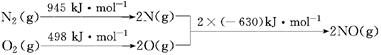
д���÷�Ӧ���Ȼ�ѧ����ʽ��________________��
��2��������(CH3OCH3)����ɫ���壬����Ϊһ��������Դ���ɺϳ���(���ΪH2��CO��������CO2)ֱ���Ʊ������ѣ����е���Ҫ���̰��������ĸ���Ӧ��
�״��ϳɷ�Ӧ��
(��)CO(g)��2H2(g)=CH3OH(g)��H1����90.1 kJ��mol��1
(��)CO2(g)��3H2(g)=CH3OH(g)��H2O(g)��H2����49.0 kJ��mol��1
ˮú���任��Ӧ��
(��)CO(g)��H2O(g)=CO2(g)��H2(g)��H3����41.1 kJ��mol��1
�����Ѻϳɷ�Ӧ��
(��)2CH3OH(g)=CH3OCH3(g)��H2O(g)��H4����24.5 kJ��mol��1
��H2��COֱ���Ʊ�������(��һ����Ϊˮ����)���Ȼ�ѧ����ʽΪ_______________��
���ݻ�ѧ��Ӧԭ������������ѹǿ��ֱ���Ʊ������ѷ�Ӧ��Ӱ��_________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���ڵؿ�����Ҫ����������ε���ʽ���ڣ��䵥�ʺͻ������ڹ�ũҵ������������Ҫ��Ӧ�á�
��1����֪���ؾ�ʯ��BaSO4���������տɷ���һϵ�з�Ӧ�����в��ַ�Ӧ���£�
BaSO4(s)+4C(s)=BaS(s)+4CO(g) ��H=" +" 571.2 kJ?mol��1
BaS(s)= Ba(s)+S(s) ��H=" +460" kJ?mol��1
��֪��2C(s)+O2(g)=2CO(g) ��H=" -221" kJ?mol��1
��Ba(s)+S(s)+2O2(g)=BaSO4(s) ��H= ��
��2���ۻ�(As4S4)�ʹƻ�(As2S3)����ȡ�����Ҫ����ԭ�ϡ���֪As2S3��HNO3�����·�Ӧ��
As2S3+10H++ 10NO3-=2H3AsO4+3S+10NO2��+ 2H2O
����Ӧ��ת�Ƶ��ӵ���ĿΪ2molʱ������H3AsO4�����ʵ���Ϊ ��
��3��������ʵ���Ũ��Na2S��NaOH�����Һ�еμ�ϡ������������������Ҫ��������֣�H2S��HS����S2�����ķֲ�������ƽ��ʱij���ֵ�Ũ��ռ������Ũ��֮�͵ķ�������μ���������Ĺ�ϵ����ͼ��ʾ�����Եμӹ���H2S������ݳ�����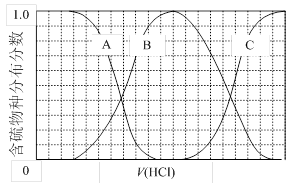
�ٺ�������B��ʾ ���ڵμ���������У���Һ��c(Na+)�뺬�������Ũ�ȵĴ�С��ϵΪ (����ĸ)��
a��c(Na+)= c(H2S)+c(HS��)+2c(S2��)
b��2c(Na+)=c(H2S)+c(HS��)+c(S2��)
c��c(Na+)=3[c(H2S)+c(HS��)+c(S2��)]
��NaHS��Һ�ʼ��ԣ�������Һ�м���CuSO4��Һ��ǡ����ȫ��Ӧ��������Һ��ǿ���ԣ���ԭ���� �������ӷ���ʽ��ʾ����
��4������л��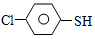 �����ȩ���Ȼ��������ʵ���֮��1:1:1��Ӧ���ɻ��һ��ɱ����м���X��H2O��
�����ȩ���Ȼ��������ʵ���֮��1:1:1��Ӧ���ɻ��һ��ɱ����м���X��H2O�� ��X�ĺ˴Ź�����������ͼ������ �����Ϊ
��X�ĺ˴Ź�����������ͼ������ �����Ϊ �ĺ˴Ź�������ͼ��д��X�Ľṹ��ʽ�� ��
�ĺ˴Ź�������ͼ��д��X�Ľṹ��ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
һ�������£������Ϊ3 L���ܱ������з�Ӧ��CO��g��+ 2H2��g�� CH3OH��g���ﵽ��ѧƽ��״̬��
CH3OH��g���ﵽ��ѧƽ��״̬��
��1���÷�Ӧ��ƽ�ⳣ������ʽK= ��������ͼ�������¶ȣ�Kֵ�� �����������С�����䡱����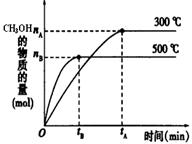
��2��500��ʱ���ӷ�Ӧ��ʼ���ﵽ��ѧƽ�⣬��H2��Ũ�ȱ仯��ʾ�Ļ�ѧ��Ӧ������ ����nB��tB��ʾ����
��3���жϸÿ��淴Ӧ�ﵽ��ѧƽ��״̬�ı�־�� ������ĸ����
a��CO��H2��CH3OH��Ũ�Ⱦ����ٱ仯
b�����������ܶȲ��ٸı�
c����������ƽ����Է����������ٸı�
d��v������CH3OH��= v������CO��
��4��300��ʱ�����������ݻ�ѹ����ԭ����1/2���������������������£���ƽ����ϵ������Ӱ���� ������ĸ����
a��c��H2������
b������Ӧ���ʼӿ죬�淴Ӧ���ʼ���
c��CH3OH �����ʵ�������
d������ƽ��ʱc��H2��/ c��CH3OH����С
��5��������Ŀ�й���Ϣ��������������ͼ�б�ʾ���û�ѧ��Ӧ���̵������仯��������Ϣ����
��6���Լ״�������������������ҺΪԭ�ϣ�ʯīΪ�缫�ɹ���ȼ�ϵ�ء���֪��ȼ�ϵ�ص��ܷ�ӦʽΪ��2CH3OH +3O2+4OH- = 2CO32- + 6H2O���õ���и����ϵĵ缫��Ӧʽ�ǣ�2CH3OH�C12e��+16OH���� 2CO32��+ 12H2O ���������Ϸ����ĵ缫��ӦΪ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ԴΣ���ǵ�ǰȫ�����⣬��Դ������Ӧ����ԴΣ������Ҫ�ٴ롣
(1)����������������Դ����Դ����������________(�����)��
a��������չũ���������������Ľո�ת��Ϊ����Ч����Դ
b����������ú��ʯ�ͺ���Ȼ������������������������Դ����
c������̫���ܡ�ˮ�ܡ����ܡ������ܵ�����Դ������ʹ��ú��ʯ�͵Ȼ�ʯȼ��
d��������Դ���ģ�������Դ���ظ�ʹ�á���Դ��ѭ������
(2)���ʯ��ʯī��Ϊ̼��ͬ�������壬����ȼ����������ʱ����һ����̼�����ȼ�����ɶ�����̼����Ӧ�зų���������ͼ��ʾ��
����ͨ��״���£����ʯ��ʯī��________(����ʯ����ʯī��)���ȶ���ʯī��ȼ����Ϊ________��
��12 gʯī��һ����������ȼ�գ���������36 g���ù��̷ų�������________��
(3)��֪��N2��O2�����л�ѧ���ļ��ֱܷ���946 kJ��mol��1��497 kJ��mol��1��
N2(g)��O2(g)=2NO(g)����H��180.0 kJ��mol��1��
NO�����л�ѧ���ļ���Ϊ________kJ��mol��1��
(4)�ۺ������й���Ϣ����д��CO��NO��Ӧ���Ȼ�ѧ����ʽ_________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����ʵ��Ļ�ѧ������ա�
��1��Na2CO3ˮ������ӷ���ʽ�� ��
��2��H2S���뷽��ʽ�� ��
��3��AlCl3ˮ������ӷ���ʽ�� ��
��4����25�桢101 kPa�£�l g������ȫȼ������CO2��Һ̬ˮʱ����55��6 kJ������д����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽ�� ��
��5����������ȼ�ϵ�ص������缫����ʽ
������ ��
������ ��
��6��д��NaHCO3��Һ�е�����Ũ�ȹ�ϵ
c(H+)+c(Na+)= ��
c(Na+)= ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com