
�ྦྷ�裨�赥�ʵ�һ�֣�����Ϊ�����Ӵ��õĻ�ʯ�����Ʊ��и�������SiCl4Ϊ������������Ⱦ�ܴ�����ˮǿ��ˮ�⣬�ų��������ȡ��о���Ա����SiCl4ˮ�����ɵ�����ͱ���ۣ���Ҫ�ɷ�ΪBaCO3���Һ�������þ�����ӣ��Ʊ�BaCl2��2H2O�������������£�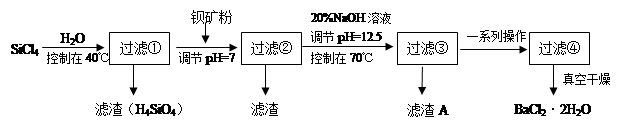
��֪��
�ٳ�����Fe3+��Mg2+��ȫ������pH�ֱ���3.4��12.4
��BaCO3����Է���������197��BaCl2��2H2O����Է���������244
�ش��������⣺
��1��SiCl4����ˮ�ⷴӦ�Ļ�ѧ����ʽΪ_______________________________________
��2����H2��ԭSiCl4��������ȡ���ȺܸߵĹ裬����Ӧ����1mol����ת��ʱ����59KJ��������÷� Ӧ���Ȼ�ѧ����ʽΪ_____________________________________________
��3���ӱ���۲�����pH=7��Ŀ���Ǣ� ����
��4�����ˢں����Һ��Fe3+Ũ��Ϊ ����Һ�¶�25�棬Ksp[Fe(OH)3]=2.2��10-38��
��5����������A�����ӷ���ʽ__________________________________________
��6����ʽ�����10�ֺ�78.8% BaCO3�ı�������������������BaCl2��2H2O������Ϊ���ٶ֣�
(14�֣���2��6�ʸ�3�֣�������2��)��1��SiCl4+4H2O��H4SiO4��+4HCl
��2��SiCl4(s)+2H2(g)��Si(s)+4HCl(s) ��H��+236kJ/mol
��3��ʹBaCO3ת��ΪBaCl2��ʹFe3+��ȫ���� ��4��2.2��10-17mo/L
��5��Mg2����2OH����Mg(OH)2�� ��6��= ��244��9.76t
��244��9.76t
���������������1���Ȼ���ˮ������ԭ������Ȼ��⣬ˮ�ⷽ��ʽΪSiCl4+4H2O��H4SiO4��+4HCl��
��2���ڷ�Ӧ�й�Ԫ�صĻ��ϼ۴ӣ�4�۽��͵�0�ۣ��õ�4�����ӡ�����Ӧ����1mol����ת��ʱ����59KJ������������1mol�Ȼ��������յ�������59kJ��4��236kJ����˸÷�Ӧ���Ȼ�ѧ����ʽΪSiCl4(s)+2H2(g)��Si(s)+4HCl(s) ��H��+236kJ/mol��
��3��pH��3.4ʱ��������������ȫ���ɳ����������̼�ᱵ��Ӧ�����Ȼ����Ͷ�����̼��ˮ�����Լӱ���۲�����pH��7��������ʹBaCO3ת��ΪBaCl2��ͬʱʹFe3+��ȫ������
��4����Һ��pH��7������Һ�� c(OH��)��1��10��7mo/L���������������ܶȻ�������֪����Һ��Fe3+Ũ�ȣ�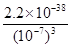 ��2.2��10-17mo/L��
��2.2��10-17mo/L��
��5��������Һ�л�����þ���ӣ�������Ҫ������Һ��pH��12.5��ʹþ������ȫ��������������þ����Ӧ�����ӷ���ʽ��Mg2����2OH����Mg(OH)2��
��6�����ݱ�ԭ���غ��֪��10�ֺ�78.8% BaCO3�ı�������������������BaCl2��2H2O������Ϊ ��244��9.76t��
��244��9.76t��
���㣺�����Ʊ�ʵ�鷽������ƣ��Ȼ�ѧ����ʽ����д�����ܵ���ʵ��ܽ�ƽ�⼰����ת���ļ��㣻�����Ʊ��ļ����


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������ʹ�������Դ��չ����̼���á�������Ϊ��ѧ���о�����Ҫ���⡣�������״������ʵ����ȼ�ϣ�������ȼ�ϵ�ء�
��1����֪���� 2CH3OH(1) + 3O2(g) = 2CO2(g) + 4H2O(g) ��H1 =" �C" 1275.6 kJ/mol
�� 2CO(g) + O2(g) = 2CO2(g) ��H2 =" �C" 566.0 kJ/mol
�� H2O(g) = H2O(1) ��H3 =" �C" 44.0 kJ/mol
д���״�����ȫȼ������һ����̼��Һ̬ˮ���Ȼ�ѧ����ʽ��___________��
��2�������״���ԭ��CO��H2��Դ�ڣ�CH4(g) + H2O(g)  CO(g) + 3H2(g) ��H>0
CO(g) + 3H2(g) ��H>0
��һ��������CH4��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼa����Tl ________T2(�<������>������=������ͬ)��A��B��C���㴦��Ӧƽ�ⳣ����KA��KB��KC���Ĵ�С��ϵΪ___________��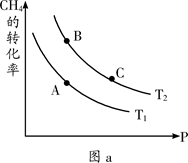
��100��ʱ����1 mol CH4��2 mol H2Oͨ���ݻ�Ϊ1 L�Ķ����ܷ������У�������Ӧ����˵���÷�Ӧ�Ѿ��ﵽƽ��״̬����__________
a�������������ܶȺ㶨
b����λʱ��������0.1 mol CH4ͬʱ����0.3 mol H2
c��������ѹǿ�㶨
d��3v��(CH4) = v��(H2)
����ﵽƽ��ʱCH4��ת����Ϊ0.5����100��ʱ�÷�Ӧ��ƽ�ⳣ��K =___________
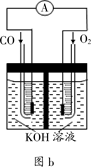
��3��ijʵ��С������CO(g) �� O2(g) ��KOH��aq����Ƴ���ͼb��ʾ�ĵ��װ�ã���õ�ظ����ĵ缫��ӦʽΪ___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
��14�֣��о������仯���������Ҫ���塣
��l����֪��
Ag2O��s��+2HC1��g�� 2AgC1��s��+H2O��1�� ��H1=��324��4 kJ��mo1��1
2Ag��s��+1/2O2��g�� Ag2O��s�� ��H2=��30��6 kJ��mo1��1
H2��g��+C12��g�� 2HC1��g�� ��H3=��184��4 kJ����mo1��1
2H2��9��+O2��g�� 2H2O��1�� ��H4=��571��2 l��mo1��1
д�������������ɹ����Ȼ������Ȼ�ѧ����ʽ________��
��2�����������γ���Fe(NO3)3��Һʴ�̣�д��Fe3+��Ag��Ӧ�����ӷ���ʽ___ _��Ҫ�ж�Fe(NO3)3��Һ��NO3���Ƿ�������ʴ���з�����Ӧ����ȡ ��������Һ��Ȼ��������Ƿ���Ag������Ӧ���ж���
��3����п���Ե�صĵ������ҺΪKOH��Һ���ŵ�ʱ������Ag2O2ת��ΪAg������Znת��ΪZn(OH)2����������ӦʽΪ ������������Һ��pH ___ ������������䡱��С������
��4����ⷨ������ʱ������Ӧ��ֱ����Դ�� ������������AgNO3��HNO3�����Һ���������Һʱ��������������������ɫ���壬�����������ĵ缫��ӦʽΪ____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
������������Ȼ����Ϊԭ�Ϻϳɼ״������ⱻһһ���ˣ�����شٽ��˼״���ѧ�ķ�չ��
��1����̿��ˮ�����ķ�Ӧ���ƣ�����Ȼ��Ϊԭ��Ҳ�����Ƶ�CO��H2���÷�Ӧ�Ļ�ѧ����ʽΪ_________��
��2���ϳɼ״���һ�ַ�������CO��H2Ϊԭ�ϣ��������仯��ͼ��ʾ��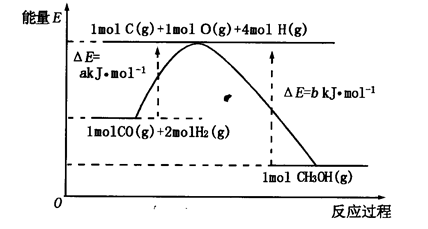
��ͼ��֪���ϳɼ״����Ȼ�ѧ����ʽΪ________________________________________��
��3����CO2Ϊԭ��Ҳ���Ժϳɼ״����䷴Ӧԭ��Ϊ��CO2(g)+3H2(g) CH3OH(g)+H2O(g)
CH3OH(g)+H2O(g)
����lL���ܱ������У�����1molCO2��3molH2����500���·�����Ӧ�����CO2(g)��CH3OH(g)��Ũ����ʱ�ʱ仯��ͼ��ʾ��
������˵����ȷ����_________________(����ĸ)��
| A��3minʱ��Ӧ�ﵽƽ�� |
| B��0��10minʱ��H2��ʾ�ķ�Ӧ����Ϊ0��225mol��-1��min-1 |
| C��CO2��ƽ��ת����Ϊ25�� |
D�����¶�ʱ��ѧƽ�ⳣ��Ϊ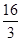 ��mol/L����2 ��mol/L����2 |
| ���� | ����1 | ����2 | ����3 |
| ��Ӧ��Ͷ������ʼ̬�� | 1molCO2��3molH2 | 0.5molCO2��1.5molH2 | 1molCH3OH��1molH2O |
| CH3OH��ƽ��Ũ��/mol?L-1 | c1 | c2 | c3 |
| ƽ��ʱ��ϵѹǿ/Pa | p1 | p2 | p3 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��ҵ�ϲ����ұ���CO2����������Ҫ����ԭ�ϱ���ϩ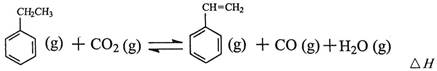
�����ұ���CO2�����еķ�Ӧ�ɷ���������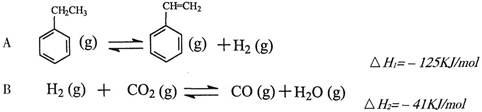
��1�������ұ���CO2��Ӧ�ķ�Ӧ�ȡ�HΪ________________________��
��2�����ұ���CO2��Ӧ��ƽ�ⳣ������ʽΪ��K=______________________��
��������������˵���ұ���CO2��Ӧ�Ѵﵽƽ��״̬����_____________________��
a��v��(CO)=v��(CO) b��c(CO2)=c(CO)
c������1mol CO2ͬʱ����1molH2O d��CO2������������ֲ���
��3����3L�ܱ������ڣ��ұ���CO2�ķ�Ӧ�����ֲ�ͬ�������½���ʵ�飬�ұ���CO2����ʼŨ�ȷֱ�Ϊ1.0mol/L��3.0mol/L������ʵ��I��T1�桢0.3MPa����ʵ��II��III�ֱ�ı���ʵ�������������ұ���Ũ����ʱ��ı仯��ͼI��ʾ��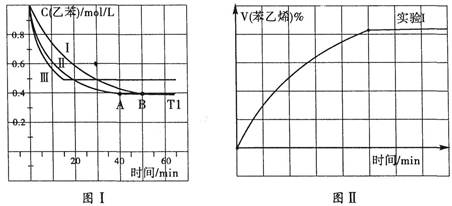
��ʵ��I�ұ���0��50minʱ�ķ�Ӧ����Ϊ_______________��
��ʵ��II���ܸı���������__________________________��
��ͼII��ʵ��I�б���ϩ�������V%��ʱ��t�ı仯���ߣ�����ͼII�в���ʵ����б���ϩ�������V%��ʱ��t�ı仯���ߡ�
��4����ʵ��I�н��ұ�����ʼŨ�ȸ�Ϊ1.2mol/L�������������䣬�ұ���ת���ʽ������������С�����䡱���������ʱƽ�ⳣ��Ϊ_____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
Ŀǰ��ҵ�ϳɰ���ԭ���ǣ�N2(g)+3H2(g) 2NH3(g) ��H=��93.0kJ /mol�����ݱ�����һ�������£�2N2(g)+6H2O(l)
2NH3(g) ��H=��93.0kJ /mol�����ݱ�����һ�������£�2N2(g)+6H2O(l) 4NH3(g)+3O2(g) ��H=" +1530.0kJ" /mol��
4NH3(g)+3O2(g) ��H=" +1530.0kJ" /mol��
��1��������ȼ���ȡ�H=_______________kJ/mol��
��2���ں��º�ѹװ���н��й�ҵ�ϳɰ���Ӧ������˵����ȷ���� ��
| A������������ٱ仯������ƽ�� |
| B�������ܶȲ��ٱ仯����δƽ�� |
| C��ƽ�����װ����ͨ��һ����Ar��ѹǿ���䣬ƽ�ⲻ�ƶ� |
| D��ƽ���ѹ��װ�ã����ɸ���NH3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
һ�������£�ͨ�����з�Ӧ��ʵ��ȼú��������Ļ��գ�
2CO(g)+SO2(g) 2CO2(g)+S(l) ��H
2CO2(g)+S(l) ��H
��1����֪2CO(g)+O2(g)= 2CO2(g) ��H1=��566kJ?mol��1
S(l) +O2(g)= SO2(g) ��H2=��296kJ?mol��1
��Ӧ�Ȧ�H= kJ?mol��1��
��2������������ͬ��������ͬʱ��SO2��ת�����淴Ӧ�¶ȵı仯��ͼa��260��ʱ ����Fe2O3��NiO��Cr2O3����������Ӧ������졣Fe2O3��NiO����������ʹSO2��ת���ʴﵽ��ߣ������Ǽ۸����أ�ѡ��Fe2O3����Ҫ�ŵ��� ��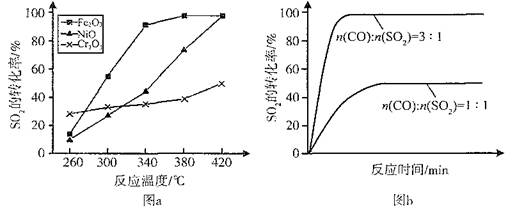
��3������С����380�桢Fe2O3������ʱ���о��˲�ͬͶ�ϱ�[n(CO)��n(SO2)]��SO2ת���ʵ�Ӱ�죬�����ͼb�����ڴ������ͼ�л���n(CO)��n(SO2)="2��1" ʱ��SO2ת���ʵ�Ԥ�ڱ仯���ߡ�
��4����ҵ�ϻ�����Na2SO3��Һ���������е�SO2��Na2SO3+SO2+H2O=2NaHSO3��ij�¶�����1.0mol?L��1 Na2SO3��Һ���մ�����SO2������Һ��c(SO32��)����0.2mol?L��1ʱ���������������½���Ӧ�������ռ���
�ٴ�ʱ��Һ��c(HSO3��)ԼΪ______mol?L��1��
�ڴ�ʱ��ҺpH=______������֪���¶���SO32��+H+ HSO3����ƽ�ⳣ��K="8.0" �� 106 L?mol��1������ʱSO2��H2SO3��Ũ�Ⱥ��Բ��ƣ�
HSO3����ƽ�ⳣ��K="8.0" �� 106 L?mol��1������ʱSO2��H2SO3��Ũ�Ⱥ��Բ��ƣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ͼ��ʾ�����Թܷ���ʢ��25 ��ı��ͳ���ʯ��ˮ���ձ��У��Թܿ�ʼ���뼸С��þƬ�����õιܵ���5 mL�������Թ��У��Իش��������⣺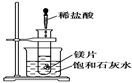
��.��1��ʵ���й۲쵽��������
________________________________________________________________________
________________________________________________________________________
��2���������������ԭ����
________________________________________________________________________
________________________________________________________________________
��3��д���йط�Ӧ�����ӷ���ʽ
________________________________________________________________________
��4����ʵ����֪��MgCl2��Һ��H2��������________(����ڡ�����С�ڡ����ڡ�)þƬ���������������
��.��֪�Ͽ�1mol H��H����1mol N��H����1 molNN���ֱ���Ҫ���յ�����Ϊ436kJ��391kJ��946kJ��һ���������������͵�����Ӧ����1mol NH3��Ҫ________(��ų��������ա�)________kJ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��һ�������£���ӦN2+3H2 2NH3����2L�ܱ������н��У�5min�ڰ�������������1.7g����Ӧ����Ϊ��
2NH3����2L�ܱ������н��У�5min�ڰ�������������1.7g����Ӧ����Ϊ��
| A��v��NH3��="0.1" mol����L��min�� | B��v��N2��="0.02" mol����L��min�� |
| C��v��H2��="0.015" mol����L��min�� | D��v��NH3��="0.17" mol����L��min�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com