
A��B��C��D��E����ѧ��ѧ�г�������ɫ���壬���Ǿ��ɶ�����Ԫ����ɡ�A��B��C�ת���Ĺ�ϵ��ͼ��ʾ(���ֲ�������ȥ)��
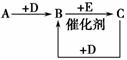
��A��ʹʪ��ĺ�ɫʯ����ֽ������C��DΪ�����е���Ҫ�ɷ֣�B��E���ж����壬���ǵķ��Ӿ���ͬ����Ԫ��ԭ�ӹ��ɡ�
��B��D�������ɺ���ɫ���塣
��ش��������⣺
(1)C�ĵ���ʽ��________��
(2)д��A��B��Ӧ�Ļ�ѧ����ʽ��____________________________________��
(3)B��E������Ӧ�Ļ�ѧ����ʽ��__________________________ ___________________________________________________��
(4)�����£���һ������Aͨ��ˮ�У������Һ��pH��12�������Һ����ˮ�������OH����Ũ��Ϊ________������Һ�У�һ���ܴ����������������________(�����)��
a��Na����NO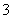 ��Fe2����Cl��
��Fe2����Cl��
b��Ag����Mg2����Ca2����NO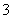
c��Al3����K����AlO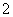 ��Cl��
��Cl��
d��CO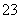 ��Na����K����NO
��Na����K����NO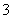
��������A��ʹʪ��ĺ�ɫʯ����ֽ��������AΪNH3,4NH3��5O2 4NO��6H2O��B��E����ͬ����Ԫ��ԭ�ӹ��ɵ��ж����壬������Ϣ�ڿ�֪��BΪNO��EΪCO������2NO��2CO
4NO��6H2O��B��E����ͬ����Ԫ��ԭ�ӹ��ɵ��ж����壬������Ϣ�ڿ�֪��BΪNO��EΪCO������2NO��2CO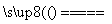 N2��2CO2��N2��O2
N2��2CO2��N2��O2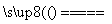 2NO����CΪN2��DΪO2��(1)CΪN2��������д���N��N���ɴ˿���д����ʽ��(2)A��B��ΪNH3��NO��ΪNH3�Ĵ���������Ҫ�����ͼ��ȡ�(3)BΪNO��EΪCO�����������ΪC(N2)����NO���������ԣ�CO���ֻ�ԭ������CO2��(4)NH3����ˮ����NH3��H2O������Һ�е�H������H2O�����������c(H��)��c(H��)H2O��c(OH��)H2O��1.0��10��12 mol��L��1������Fe2����2NH3��H2O===Fe(OH)2����2NH
2NO����CΪN2��DΪO2��(1)CΪN2��������д���N��N���ɴ˿���д����ʽ��(2)A��B��ΪNH3��NO��ΪNH3�Ĵ���������Ҫ�����ͼ��ȡ�(3)BΪNO��EΪCO�����������ΪC(N2)����NO���������ԣ�CO���ֻ�ԭ������CO2��(4)NH3����ˮ����NH3��H2O������Һ�е�H������H2O�����������c(H��)��c(H��)H2O��c(OH��)H2O��1.0��10��12 mol��L��1������Fe2����2NH3��H2O===Fe(OH)2����2NH ��Ag����NH3��H2O===AgOH����NH
��Ag����NH3��H2O===AgOH����NH ��Al3����3AlO
��Al3����3AlO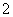 ��6H2O===4Al(OH)3����Al3����3NH3��H2O===Al(OH)3����3NH
��6H2O===4Al(OH)3����Al3����3NH3��H2O===Al(OH)3����3NH ��ȷ��dΪ��ȷѡ�
��ȷ��dΪ��ȷѡ�
�𰸡�(1)��N⋮⋮N�á�(2)4NH3��5O2 4NO��6H2O��(3)2NO��2CO
4NO��6H2O��(3)2NO��2CO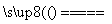 N2��2CO2��(4)1��10��12 mol��L��1��d
N2��2CO2��(4)1��10��12 mol��L��1��d



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����¶ȣ��������ݲ�һ��������� �� ��
A����ѧ��Ӧ����v B��������ʵĵ���ƽ�ⳣ��Ka
C����ѧƽ�ⳣ��K D��ˮ�����ӻ�����KW
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ϳ�ϴ�Ӽ�ͬ�����������Щ�ŵ㣺��ԭ�ϱ��ˡ��ڲ�����ˮ��Ӳˮ�����ơ��۲���Ⱦˮ�塡��ȥ������ǿ������Ϊ��ȷ���� (����)��
A���٢ڢ� B���ڢۢ� C���٢� D���٢ۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������������Ҫ�Ĺ�ҵԭ�ϣ�̽�����Ʊ����������ʾ��зdz���Ҫ�����塣
(1)��ҵ���û�����(FeS2��������Ԫ��Ϊ��1��)�ڸ����º�������Ӧ�Ʊ�SO2��4FeS2��11O2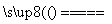 8SO2��2Fe2O3���÷�Ӧ�б�������Ԫ����________(��Ԫ�ط���)�����÷�Ӧת��2.75 mol����ʱ�����ɵĶ��������ڱ�״���µ����Ϊ________ L��
8SO2��2Fe2O3���÷�Ӧ�б�������Ԫ����________(��Ԫ�ط���)�����÷�Ӧת��2.75 mol����ʱ�����ɵĶ��������ڱ�״���µ����Ϊ________ L��
(2)ʵ������������װ�òⶨSO2������ΪSO3��ת���ʡ�(��֪SO3���۵�Ϊ16.8 �棬�����������װ��ʱ�ֱ���ȫ���գ��Һ��Կ�����CO2��Ӱ��)
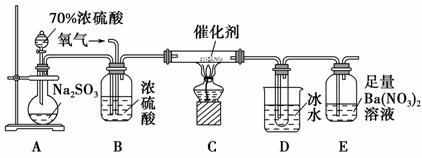
�ټ���ʹ�÷�Һ©����Բ����ƿ�еμ�Ũ����IJ�����________________________________________��
��ʵ������У���Ҫͨ����������д��һ��������ͼ��ʾװ����ȡ�����Ļ�ѧ����ʽ��______________________________________��

�۵�ֹͣͨ��SO2��Ϩ��ƾ��ƺ���Ҫ����ͨһ��ʱ�����������Ŀ����___________________________________________________________��
��ʵ���������װ��D���ӵ�����Ϊm g��װ��E�в�����ɫ����������Ϊn g����������¶��������ת������________(�ú���ĸ�Ĵ���ʽ��ʾ�����û���)��
(3)ijѧϰС���������ͼװ����֤��������Ļ�ѧ���ʡ�
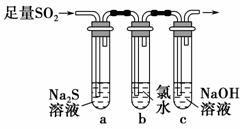
����˵������������������Ե�ʵ������Ϊ_______________________ ________________________________________________________��
��Ϊ��֤��������Ļ�ԭ�ԣ���ַ�Ӧ��ȡ�Թ�b�е���Һ�ֳ����ݣ��ֱ��������ʵ�顣
���������һ����Һ�м���AgNO3��Һ���а�ɫ��������
��������ڶ�����Һ�м���Ʒ����Һ����ɫ��ȥ
���������������Һ�м���BaCl2��Һ��������ɫ����
���������к�������________(�������)���Թ�b�з�����Ӧ�����ӷ���ʽΪ____________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ϊ�˼���ij�����������Ƿ���NH ��������ֽ���Լ�һ���ò������� (����)��
��������ֽ���Լ�һ���ò������� (����)��
������ˮ����NaOH��Һ���ۺ�ɫʯ����ֽ������ɫʯ����ֽ����ϡ����
A���٢ݡ� B���ܢݡ�
C���٢ۡ� D���٢ܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ӵĽṹʾ��ͼ������ȷ���� (����)��
A��F���Ľṹʾ��ͼ��
B��S2���Ľṹʾ��ͼ��
C��Na���Ľṹʾ��ͼ��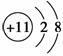
D��Cl���Ľṹʾ��ͼΪ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��ʾ��a��b��c��Ϊ�ǽ������ʣ�d��e��Ϊ����10�����ӵĹ��ۻ�����ҷ���������ԭ�Ӹ�����d��e��fΪ���ӻ����������˵��������� (����)��

A�������£�����a����̬�� B������c����ǿ������
C���ȶ��ԣ�d��e�� D��f�����ֽ�Ϊd��e
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ�������£������Ա����������Ļ�����������ɶ����������1,5��������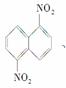 1,8��������
1,8��������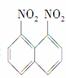 �Ļ������߿�����������������98%�����ᣬ��ǰ�߲��ܡ�������һ���ʿɽ�������ͬ���칹����롣���������������������98%�����ᣬ��ֽ��裬������©�����ˣ�������Һ�еõ�����1,8����������Ӧ���õķ�����(����)
�Ļ������߿�����������������98%�����ᣬ��ǰ�߲��ܡ�������һ���ʿɽ�������ͬ���칹����롣���������������������98%�����ᣬ��ֽ��裬������©�����ˣ�������Һ�еõ�����1,8����������Ӧ���õķ�����(����)
A������Ũ���ᾧ B������Һ�м�ˮ����
C����̼������Һ������Һ D������Һ��������ˮ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ˮ������������Դ���ҹ��ǵ�ˮ��Դ��ƶ���Ĺ���֮һ����Լ��ˮ�ѳ�Ϊȫ������Ĺ�ʶ�����в����Ͻ�Լ��ˮ�������� (����)��
A��ũ���ռ��ι༼��
B�������������ɵ���ˮ���Բ������ˮ��Դ��ȱ
C��������ˮ���������ڳ����̻���ũҵ���
D��������ȾԴ������ˮԴ�ز�����Ⱦ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com