
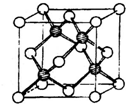
 ¢ń£®£Ø1£©Ģ¼ŹĒŠĪ³É»ÆŗĻĪļ×ī¶ąµÄŌŖĖŲ£¬Ę䵄֏Óė»ÆŗĻĪļ¹ć²¼ÓŚ×ŌČ»½ē£®CS2ŹĒŅ»ÖÖÖŲŅŖµÄÓŠ»śČܼĮ£¬Ęä½į¹¹ÓėCO2ĻąĖĘ£¬CS2·Ö×ÓÖŠÖŠŠÄŌ×ÓµÄŌÓ»ÆĄąŠĶĪŖ
¢ń£®£Ø1£©Ģ¼ŹĒŠĪ³É»ÆŗĻĪļ×ī¶ąµÄŌŖĖŲ£¬Ę䵄֏Óė»ÆŗĻĪļ¹ć²¼ÓŚ×ŌČ»½ē£®CS2ŹĒŅ»ÖÖÖŲŅŖµÄÓŠ»śČܼĮ£¬Ęä½į¹¹ÓėCO2ĻąĖĘ£¬CS2·Ö×ÓÖŠÖŠŠÄŌ×ÓµÄŌÓ»ÆĄąŠĶĪŖ| 1 |
| 8 |
| 1 |
| 2 |
| 1 |
| 2 |
| 1 |
| 2 |


 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 £»
£»²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
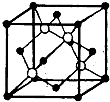 £Ø2013?ĮŁŅŹŅ»Ä££©”¾»Æѧ--ĪļÖŹ½į¹¹ÓėŠŌÖŹ”æ
£Ø2013?ĮŁŅŹŅ»Ä££©”¾»Æѧ--ĪļÖŹ½į¹¹ÓėŠŌÖŹ”æ| 160 |
| a3b |
| 160 |
| a3b |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2008-2009Ń§ÄźÉ½¶«Ź”ĒąµŗŹŠø߶ž£ØĻĀ£©ĘŚÄ©»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013ÄźÉ½¶«Ź”ĮŁŅŹŹŠøßæ¼»ÆѧŅ»Ä£ŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com