
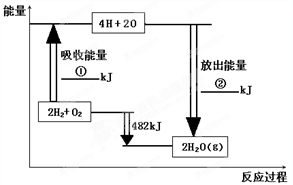
 ��֪��1mol�����еĻ�ѧ����Ҫ����436kJ��������1mol�����еĻ�ѧ����Ҫ����498kJ����������ͼ�е�����ͼ���ش��������⣺
��֪��1mol�����еĻ�ѧ����Ҫ����436kJ��������1mol�����еĻ�ѧ����Ҫ����498kJ����������ͼ�е�����ͼ���ش��������⣺| 1852kJ |
| 4 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ���㽭�����������ѧУ��һ��ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ������
Ϊ�˺������û�ѧ�ܣ�ȷ����ȫ���������������Ҫ��ֿ��ǻ�ѧ��Ӧ���ʱ䣬����ȡ��Ӧ�Ĵ�ʩ����ѧ��Ӧ���ʱ�һ��ͨ��ʵ����вⶨ��Ҳ�ɽ����������㡣
��1��ʵ���ã�0.3mol��̬����ȼ�������飨B2H6����������ȼ�գ����ɹ�̬�����������Һ̬ˮ���ų�649.5kJ������д������ȼ�շ�Ӧ���Ȼ�ѧ����ʽ ����״����11.2L��������ȫȼ������Һ̬ˮʱ�ų��������� kJ��
��2���ڻ�ѧ��Ӧ�����У���ѧ����Ҫ�����������γɻ�ѧ���ֻ��ͷ���������֪��1mol�����еĻ�ѧ����Ҫ����436kJ��������1mol�����еĻ�ѧ����Ҫ����496kJ�������γ�ˮ�����е�1mol H-O���ܹ��ͷ�463kJ��������˵����Ӧ2H2(g)+O2(g)=2H2O(g)�е������仯��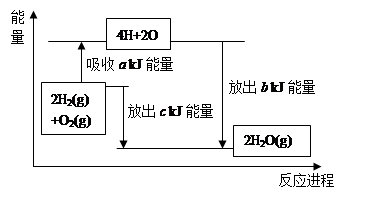
a= ��b= ����֪1molҺ̬ˮת������̬ˮ����44 kJ��������������ȫȼ������Һ̬ˮʱ���Ȼ�ѧ����ʽΪ ��
��3�����ݸ�˹���ɿ��Զ�ijЩ����ͨ��ʵ��ֱ�Ӳⶨ�Ļ�ѧ��Ӧ���ʱ�������㡣ʵ���в���ֱ�Ӳ����ʯī���������ɼ��鷴Ӧ�ķ�Ӧ�ȣ����ɲ��CH4��ʯī��H2ȼ���ȷֱ����£�
��CH4(g)+2O2(g)=CO2(g)+2H2O ��H1=��890.3 kJ?mol-1
��C(ʯī)+O2(g)= CO2(g) ��H2= ��393.5 kJ?mol-1
��H2(g)+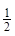 O2(g)=H2O(l) ��H3= ��285.8 kJ?mol-1
O2(g)=H2O(l) ��H3= ��285.8 kJ?mol-1
��C(ʯī)+2H2(g)= CH4(g) ��H4
�Ը��ݸ�˹�������ʯī���ɼ���ķ�Ӧ�Ȧ�H4= ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�긣��ʡ���أ��У�һ�и�һ�ڶ�ѧ����ĩ������ѧ�Ծ� ���ͣ������
��11�֣���Ч��������������Դ�Ϳ�������Դ���ܵ����������ӡ�
��1�����øĽ�������ɵİ취���������͵�ȼ�����ܡ����磬�������м����Ҵ�����������Ǧ���͡����Ҵ��ķ���ʽΪC2H6O���Ը���C��H��O�ɼ����ص㣬д��C2H6O���п��ܵĽṹʽ��ṹ��ʽ�������������������������� ��
��2����Ȼ������Ҫ�ijɷ��Ǽ��飬��ȼ�ղ���������ֵ�ߡ��ܵ����ͷ��㣬����Ϊ�ҹ������������ص�֮һ����˵�������������������������ƽ��ṹ�������� ��(��д���)
����һ��ȡ���ﲻ����ͬ���칹�� �������������������ȡ���ﲻ����ͬ���칹��
��������ȡ���ﲻ����ͬ���칹�� ��������ȡ���ﲻ����ͬ���칹��
��3������������δ����������Դ��1980���ҹ��״��Ƴ�һ��ȼ����������Ա12�ˣ���50km/h ���ٶ���ʻ��40km��Ϊ����Ч��չ��������Դ�����ȱ����Ƶ����۵����������мȿ����־��õ����ⷽ���� �������������� ����д��ţ�
���ٶ���ʻ��40km��Ϊ����Ч��չ��������Դ�����ȱ����Ƶ����۵����������мȿ����־��õ����ⷽ���� �������������� ����д��ţ�
�ٵ��ˮ ��������п��ϡ���ᷴӦ �����۹�⺣ˮ
��Σ��Ƶô���������Ҫ����������� ����д�����е�һ���� ��4��������������ȼ������ˮ���Ȼ�ѧ����ʽ
��4��������������ȼ������ˮ���Ȼ�ѧ����ʽ 2H2��g��+ O2(g) ="=" 2H2O(g) ��H��a kJ/mol
2H2��g��+ O2(g) ="=" 2H2O(g) ��H��a kJ/mol 2H2��g��+ O2(g) ==2H2O(l) ��H��b kJ/mol
2H2��g��+ O2(g) ==2H2O(l) ��H��b kJ/mol
��ش��������⣺����ʾ����b���ʾb�ľ���ֵ�� ����2 molH2��ȫȼ������ˮ��������ų����������������������������������b��kJ
����2 molH2��ȫȼ������ˮ��������ų����������������������������������b��kJ
�� ����֪��1mol�����еĻ�ѧ��Ҫ����436 kJ��������1mol�����еĻ�ѧ��Ҫ
����֪��1mol�����еĻ�ѧ��Ҫ����436 kJ��������1mol�����еĻ�ѧ��Ҫ ����496kJ������ˮ������1molH��O���γ�ʱ�ų�����463kJ��д���÷�Ӧ���Ȼ�ѧ����ʽ��
����496kJ������ˮ������1molH��O���γ�ʱ�ų�����463kJ��д���÷�Ӧ���Ȼ�ѧ����ʽ��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015���㽭�����������ѧУ��һ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ������
Ϊ�˺������û�ѧ�ܣ�ȷ����ȫ���������������Ҫ��ֿ��ǻ�ѧ��Ӧ���ʱ䣬����ȡ��Ӧ�Ĵ�ʩ����ѧ��Ӧ���ʱ�һ��ͨ��ʵ����вⶨ��Ҳ�ɽ����������㡣
��1��ʵ���ã�0.3mol��̬����ȼ�������飨B2H6����������ȼ�գ����ɹ�̬�����������Һ̬ˮ���ų�649.5kJ������д������ȼ�շ�Ӧ���Ȼ�ѧ����ʽ ����״����11.2L��������ȫȼ������Һ̬ˮʱ�ų��������� kJ��
��2���ڻ�ѧ��Ӧ�����У���ѧ����Ҫ�����������γɻ�ѧ���ֻ��ͷ���������֪��1mol�����еĻ�ѧ����Ҫ����436kJ��������1mol�����еĻ�ѧ����Ҫ����496kJ�������γ�ˮ�����е�1mol H-O���ܹ��ͷ�463kJ��������˵����Ӧ2H2(g)+O2(g)=2H2O(g)�е������仯��

a= ��b= ����֪1molҺ̬ˮת������̬ˮ����44 kJ��������������ȫȼ������Һ̬ˮʱ���Ȼ�ѧ����ʽΪ ��
��3�����ݸ�˹���ɿ��Զ�ijЩ����ͨ��ʵ��ֱ�Ӳⶨ�Ļ�ѧ��Ӧ���ʱ�������㡣ʵ���в���ֱ�Ӳ����ʯī���������ɼ��鷴Ӧ�ķ�Ӧ�ȣ����ɲ��CH4��ʯī��H2ȼ���ȷֱ����£�
��CH4(g)+2O2(g)=CO2(g)+2H2O ��H1=��890.3 kJ?mol-1
��C(ʯī)+O2(g)= CO2(g) ��H2= ��393.5 kJ?mol-1
��H2(g)+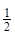 O2(g)=H2O(l)
��H3= ��285.8 kJ?mol-1
O2(g)=H2O(l)
��H3= ��285.8 kJ?mol-1
��C(ʯī)+2H2(g)= CH4(g) ��H4
�Ը��ݸ�˹�������ʯī���ɼ���ķ�Ӧ�Ȧ�H4= ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��09-10��̨���и߶���ѧ����ĩ���Ի�ѧ�� ���ͣ������
(8��)��֪��1mol�����еĻ�ѧ����Ҫ����436kJ��������1mol�����еĻ�ѧ����Ҫ����498kJ����������ͼ�е�����ͼ���ش��������⣺
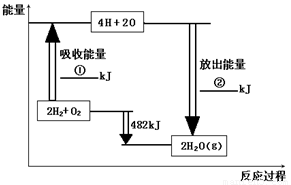
��1���ֱ�д���٢ڵ���ֵ��
�� �� �� �� �� ��
��2������H2O��g���е�1mol H-O���ų� �� kJ��������
��3����֪��H2O��l��= H2O��g�� DH = +44 kJ��mol��1 ����д����������������ȫȼ������Һ̬ˮ���Ȼ�ѧ����ʽ�� �� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com