
����Ŀ�����ڱ�ǰ�����ڵ�Ԫ��![]() ��
��![]() ��
��![]() ��
��![]() ��ԭ��������������Xԭ�ӻ�̬ʱ
��ԭ��������������Xԭ�ӻ�̬ʱ![]() ����
����![]() �����������s�����������ͬ��
�����������s�����������ͬ��![]() ԭ�ӻ�̬ʱ2pԭ�ӹ������3��δ�ɶԵĵ��ӣ�Z�ж������������һ�ֺ���ɫ���������Ϳ�ϣ�
ԭ�ӻ�̬ʱ2pԭ�ӹ������3��δ�ɶԵĵ��ӣ�Z�ж������������һ�ֺ���ɫ���������Ϳ�ϣ�![]() λ�ڵ������ڣ���ԭ�������ֻ��1�����ӣ����ڲ㶼����ȫ����״̬���ش��������⣺
λ�ڵ������ڣ���ԭ�������ֻ��1�����ӣ����ڲ㶼����ȫ����״̬���ش��������⣺
(1)Xλ�����ڱ��ĵ�_______���ڣ���______�塣
(2)Ԫ�صĵ�һ�����ܣ�X______Y(�������������ͬ)��ԭ�Ӱ뾶��X______Y��
(3)![]() ������������Ӧˮ������������ӵĿռ乹����_______(����������)��
������������Ӧˮ������������ӵĿռ乹����_______(����������)��
(4)![]() ��̬��������Ų�ʽΪ_________�������軯����Һ����
��̬��������Ų�ʽΪ_________�������軯����Һ����![]() �����ӷ���ʽΪ___________��
�����ӷ���ʽΪ___________��
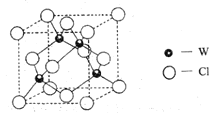
(5)Ԫ��W��һ���Ȼ��ᄃ��ľ����ṹ��ͼ��ʾ�����Ȼ���Ļ�ѧʽ��_______��������Ũ���ᷢ����������ԭ��Ӧ�����������![]() ����Ӧ�Ļ�ѧ����ʽ��_________��
����Ӧ�Ļ�ѧ����ʽ��_________��
���𰸡��� IVA �� �� ƽ�������� ![]() ��
��![]()
![]()
![]()
![]()
��������
ǰ������Ԫ��![]() ��
��![]() ��
��![]() ��
��![]() ��ԭ��������������Xԭ�ӻ�̬ʱ
��ԭ��������������Xԭ�ӻ�̬ʱ![]() ����
����![]() �����������s�����������ͬ���������Ų�ʽΪ��1s22s22p2����XΪCԪ�أ�
�����������s�����������ͬ���������Ų�ʽΪ��1s22s22p2����XΪCԪ�أ�![]() ԭ�ӻ�̬ʱ2pԭ�ӹ������3��δ�ɶԵĵ��ӣ��������Ų�ʽΪ��1s22s22p3����YΪNԪ�أ�Z�ж������������һ�ֺ���ɫ���������Ϳ�ϣ���ZΪFeԪ�أ�
ԭ�ӻ�̬ʱ2pԭ�ӹ������3��δ�ɶԵĵ��ӣ��������Ų�ʽΪ��1s22s22p3����YΪNԪ�أ�Z�ж������������һ�ֺ���ɫ���������Ϳ�ϣ���ZΪFeԪ�أ�![]() λ�ڵ������ڣ���ԭ�������ֻ��1�����ӣ����ڲ㶼����ȫ����״̬���������Ų�ʽΪ��1s22s22p63s23p63d104s1����WΪCuԪ�ء��ݴ˽��
λ�ڵ������ڣ���ԭ�������ֻ��1�����ӣ����ڲ㶼����ȫ����״̬���������Ų�ʽΪ��1s22s22p63s23p63d104s1����WΪCuԪ�ء��ݴ˽��
��1��XΪCԪ�أ�λ�����ڱ��ĵڶ����ڣ���IVA�壬�ʴ�Ϊ������IVA��
��2��XΪCԪ�أ�YΪNԪ�أ�NԪ�صĻ�̬ԭ�ӵĵ����Ų�ʽΪ��1s22s22p3��p������ڰ����״̬�����ȶ�����һ�����ܣ�C��N��ͬ���ڣ������ң�ԭ�Ӱ뾶��С��ԭ�Ӱ뾶��C��N���ʴ�Ϊ����������
��3��YΪNԪ�أ�������������Ӧˮ����ΪHNO3���������ΪNO3-������ԭ��Nԭ�ӵŶԵ�����Ϊ��![]() ���۵��Ӷ���Ϊ=
���۵��Ӷ���Ϊ=![]() ��+�¶Ե�����=0+3=3����ռ乹����ƽ�������Σ��ʴ�Ϊ��ƽ�������Ρ�
��+�¶Ե�����=0+3=3����ռ乹����ƽ�������Σ��ʴ�Ϊ��ƽ�������Ρ�
��4��ZΪFeԪ�أ�Fe3+��̬��������Ų�ʽΪ![]() ��
��![]() �������軯����Һ����Fe2+�����ӷ���ʽΪ��
�������軯����Һ����Fe2+�����ӷ���ʽΪ��![]() �����ʴ�Ϊ��
�����ʴ�Ϊ��![]() ��
��![]() ����
����
��5��WΪCuԪ�أ���ͼ��֪�������У�CuԪ�ص�ԭ�Ӹ���Ϊ4��ClԪ�ص�ԭ�Ӹ���Ϊ��![]() ������Ȼ���Ļ�ѧʽ��
������Ȼ���Ļ�ѧʽ��![]() ��
��![]() ��Ũ���ᷢ����������ԭ��Ӧ�����������
��Ũ���ᷢ����������ԭ��Ӧ�����������![]() ���䷴Ӧ�Ļ�ѧ����ʽΪ��
���䷴Ӧ�Ļ�ѧ����ʽΪ��![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��![]() ��
��


 ��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������أ�K2S2O8���ڿ����빤ҵ������Ҫ��;��
��1��S2O82-�ĽṹʽΪ[![]() ]2-������SԪ�صĻ��ϼ�Ϊ_____����Ag+���£�S2O82-��ʹ��Mn2+����Һ����Ϻ�ɫ������������_____�������ӷ��ţ���
]2-������SԪ�صĻ��ϼ�Ϊ_____����Ag+���£�S2O82-��ʹ��Mn2+����Һ����Ϻ�ɫ������������_____�������ӷ��ţ���
��2��ij������ʪ��K2S2O8���������Ͱ����������ۺϴ���ȼú��¯�������������������Ч�ʣ�����Һ��������Ϊ����ֲ���̻��ķ��ϡ�
����������У�������ҺpH=6ʱ��n(SO32-)�sn(HSO3-)=____��[��֪��25��ʱ��Ka1(H2SO3)��1.5��10-2��Ka2(H2SO3)��1.0��10-7]
���������������η���������Ӧ����1��K2S2O8��NO������HNO2����2��K2S2O8��������HNO2����2����Ӧ�Ļ�ѧ����ʽΪ________________________________��һ�������£�NOȥ�������¶ȱ仯�Ĺ�ϵ��ͼ��ʾ��80��ʱ����NO��ʼŨ��Ϊ450 mg��m-3��t min�ﵽ���ȥ���ʣ�NOȥ����ƽ����Ӧ���ʣ�v(NO) =_______mol.L-1��min-1���д���ʽ����

��3����������ؿ�ͨ��������ת�����ᴿ�������Ƶã����װ������ͼ��ʾ��

�ٵ��ʱ�����缫���ӵ�Դ��______����
�ڳ����£����Һ�к�������Ҫ������ʽ��pH�Ĺ�ϵ����ͼ��ʾ����֪������Ķ������볣��1.02��10-2���������ŵ��������Ҫ��HSO4-���������������Һ��pH��ΧΪ________�������ĵ缫��ӦʽΪ________��

��������Ʒ�м�������أ�ʹ��ת��Ϊ��������شֲ�Ʒ���ᴿ�ֲ�Ʒ�ķ�����____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£����и���������ָ����Һ���ܴ����������( )
A.��ɫ������Һ�У� Fe3+��Mg2+��SCN ��Cl
B.![]() =1��10-12����Һ�У�K+��Na+��CO32��NO3
=1��10-12����Һ�У�K+��Na+��CO32��NO3
C.![]() ����Һ�У� K+��NH4+��MnO4��SO42��
����Һ�У� K+��NH4+��MnO4��SO42��
D.��ʹ���ȱ�����Һ�У� Na+��NH4+��SO42����HCO3��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾװ�ã�������ָ�뷢��ƫת��ͬʱA����֣�B����ϸ��CΪ�������Һ����A��B��CӦ�����и����е�(����)
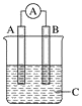
A. A��Zn��B��Cu��CΪϡ����
B. A��Cu��B��Zn��CΪϡ����
C. A��Fe��B��Ag��CΪϡAgNO3��Һ
D. A��Ag��B��Fe��CΪϡAgNO3��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����н�����ʹ�����������õ�ҩ�����ұص�����Ҫ�ɷֵĽṹ��ʽΪ
 ��������
��������
A. ������B. ������ˮ���л���C. �������ͬϵ��D. �߷��ӻ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ��С��Ӹ��� NaBr �Ĺ�ҵ��ˮ����ȡBr2 �Ĺ�����Ҫ��������������ȡ����Һ������Ȳ��衣
��֪��������ʱ����ֵķе����Խ����Ч��Խ�ã��ڿ����õ���������Ϣ��װ�����£�
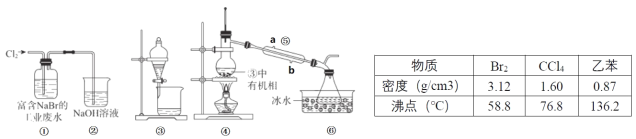
����˵���������
A.�������������ն��� Cl2����ֹ������Ⱦ
B.����������ȡʱ��ѡ���ұ��� CCl4 ������
C.�ұ��� CCl4 ��ȡ Br2 ʱ������ͬ�����Ƿֲ㣬�ϲ���ɫ���²��ɫ
D.������������Ϊ�����ܣ����� b Ϊ��ˮ�ڣ�a Ϊ��ˮ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ν���Է�����ָ�ڷ����У���һ��̼ԭ�������б˴˻�����ͬ���ĸ�ԭ�ӻ�ԭ����ʱ���ƴ˷���Ϊ���Է��ӣ�����̼ԭ��Ϊ����̼ԭ�ӡ�������һ������̼ԭ�ӵ�����һ�����й�ѧ���ԡ����磬�л�������![]() �й�ѧ���ԡ�����л�������ֱ������·�Ӧ�����ɵ��л������й�ѧ���Ե���(����)
�й�ѧ���ԡ�����л�������ֱ������·�Ӧ�����ɵ��л������й�ѧ���Ե���(����)
A. �����ᷢ��������Ӧ
B. ��NaOHˮ��Һ����
C. ��������Һ����
D. �ڴ�������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ҹ���ѧ�ҳɹ��ϳ��˺� N![]() ���嵪�������Σ�����ȫ�����ܲ����о���������̱�ʽ��ͻ�ơ�
���嵪�������Σ�����ȫ�����ܲ����о���������̱�ʽ��ͻ�ơ�
(1)N![]() �У�N ԭ��֮����������_____(�������Ӽ����������ۼ���)��
�У�N ԭ��֮����������_____(�������Ӽ����������ۼ���)��
(2)��(As)�뵪λ��ͬһ���壬�����ƶ���ȷ����_____(�����)��
�� ��Ԫ�ص�������ϼ�Ϊ3 �� �� �������������Ӧ��ˮ���������� �� ���ȶ��ԣ�AsH3��NH3
(3)�ǽ�����O ǿ��N����ԭ�ӽṹ����ԭ��_____���õ�������O ����N��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������һ�ְ�ɫ�й���Ƭ״�ᾧ���ɫ�ᾧ��ĩ���ǻǰ���ҩ���ԭ �ϣ�������ֹʹ�������ȼ�����������Ⱦ���м��塣�����������Ʊ�ԭ��Ϊ��
![]() +CH3COOH
+CH3COOH![]() +H2O
+H2O

ע�����η������������൱�ڶ����������ڷе���̫��Ļ����ķ��롣

ʵ�鲽��:
���� 1����Բ����ƿ�м�����ˮ���� 9.2 mL�������� 17.4 mL��п��0.1 g����װ�����������ʯ�����ڼ����¶ȣ�ʹ���������¶ȿ�����105�� ���ң���ӦԼ 60��80 min����Ӧ���ɵ�ˮ���������ᱻ������ ���� 2���ڽ����£����Ƚ���ƿ�е�������ϸ��״����ʢ�� 100 mL �� ˮ���ձ��У����ҽ��裬����ȴ���ᾧ�����ˡ�ϴ�ӡ�����õ����� ������Ʒ������ 3�����˴��������������ؽᾧ�����ɣ����أ�������ʡ�
(1)���� 1 ����ѡԲ����ƿ����ѹ����_________(�����)��
a��25 mL b��50 mL c��150 mL d��200 mL
(2)ʵ���м�������п�۵�Ŀ����___________________________________________________________________________��
(3)�ӻ�ѧƽ��ĽǶȷ��������Ʒ������϶˵��¶��� 105�����ҵ�ԭ��____________________________________________________________________________��
(4)ϴ������������Ʒ����ʵķ�����_____(�����)��
a����������ˮϴ b����������ˮϴ c���þƾ�ϴ
(5)����������Ʒ�����ʶ���ɫ�������ؽᾧ�����ᴿ���������£���ˮ�ܽ⡢
_______________�����ˡ�ϴ�ӡ�����(ѡ����ȷ�IJ���������)��
a�������ᾧ b����ȴ�ᾧ c�����ȹ��� d���������̿
(6)��ʵ�����յõ���Ʒ 8.1g�������������IJ�����______________ ����
(7)��ͼ��װ���� 1 ��������ָ������֮��____________________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com