
����Ŀ����ͼ��ʾ��CuSO45H2O����������480mL 0.20mol��L��1 CuSO4��Һ�ļ����ؼ�ʵ�鲽��Ͳ�������ͼ�ش��������⣺

��1��������ʵ�鲽��A��F��ʵ������Ⱥ�������� __________________�����ȡCuSO45H2O ���������Ϊ__________ g��
��2��д������480mL0.20mol��L��1CuSO4��Һ����Ҫ�õ��IJ������������ƣ��ձ�����Ͳ��_______________��
��3������Bͨ����Ϊת�ƣ���������NaOH��Һ����ˮ�ܽ�NaOH�����δ��ȴ�����¼�ת�ƣ�������Һ��Ũ�Ƚ�ƫ_____________��������������������������Aͨ����Ϊ____________��������ӿ̶��ߣ����Ƶ�Ũ�Ƚ�ƫ______����������������������
���𰸡�CBDFAE 25.0 500mol����ƿ�������� �� ���� ��
��������
��1������һ�����ʵ���Ũ����Һ�IJ��裺���㡢�������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�������Ҫ�������������m=cVM��
��2������500mLһ�����ʵ���Ũ����Һ��Ҫ��������: ������ƽ��ҩ�ס��ձ�������������ͷ�ιܡ�500mL����ƿ��
��3��������Һ��Ũ������c=![]() �����жϡ�
�����жϡ�
��1������һ�����ʵ���Ũ����Һ�IJ��裺���㡢�������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����A��F��ʵ������Ⱥ�������У�CBDFAE����Ҫ�������������m=cVM = 0.20mol/L��500mL��250g/mol =25.0g��
��2������500mLһ�����ʵ���Ũ����Һ����Ҫ�õ��IJ������������ƣ��ձ�����Ͳ��500mol����ƿ�����������ʴ�Ϊ��500mol����ƿ����������
��3������NaOH��Һ����ˮ�ܽ�NaOH�����δ��ȴ�����¼�ת�ƣ���ȴ����Һ���ƫС����ҺŨ��ƫ�ߡ�����Aͨ����Ϊ���ݣ�������ӿ̶��ߣ���Һ���ƫС�����Ƶ�Ũ�Ƚ�ƫ�ߡ��ʴ�Ϊ�������������ߡ�


 �ǻۿ����ܾ�100�ֵ�Ԫ���ؼ��ϵ�д�
�ǻۿ����ܾ�100�ֵ�Ԫ���ؼ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����0.2L��NaCl��MgCl2��BaCl2��ɵĻ����Һ�У���������Ũ�ȴ�С��ͼ��ʾ�����ڸ���Һ�ɷ֡�����˵������ȷ����( )

A. NaCl�����ʵ���Ϊ0.2mol
B. ����MgCl2������Ϊ9g
C. �û��Һ��BaCl2�����ʵ���Ϊ0.1mol
D. ���û��Һ��ˮϡ�������Ϊ1L,ϡ�ͺ���Һ��Ba2+�����ʵ���Ũ��Ϊ0.1mol/L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������ȼ�ϵ��Ϊ��Դ���е���ʵ��װ����ͼ��ʾ������˵����ȷ���ǣ� ��
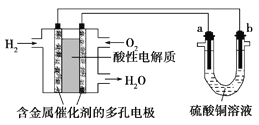
A. ȼ�ϵ�ع���ʱ��������ӦΪO2��2H2O��4e��===4OH��
B. a���Ǵ�ͭ��b���Ǵ�ͭʱ��a�����ܽ⣬b������ͭ����
C. ��������SO42�� ����b��
D. a��b��������ʯī������ͬ������a���������������������ĵ�H2������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����20��ʱ����һ�ݻ�����������ڲ���һ����©���ҿɻ����Ļ����������ָ����������ҡ����ҳ��뵪�������ҳ��������������Ļ�����壬����ǡ��ͣ��������˵�1/4��������ͼ����ʾ����Ȼ����ȼ�⡢��������壬��Ӧ��Ϻ�ָ���ԭ���¶ȣ�����ǡ��ͣ���м䣨����ͼ����ʾ�����������ˮ�����������Ӧǰ����������������ȿ�����
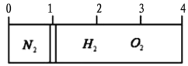

A. 3��4 B. 4��5 C. 6:2 D. 3��7
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Զ�ֲ��������Ǽ�����Ҫ�ģ���ˮ��ĵ⻯��͵����β���������������³´�л���ڸ����鶯���У����Ե⻯���������ʽ�����ڼ�״���ڣ�ȱ����������״���״�
I.��Ҫ�ӹ�ҵ�����Һ�л��յⵥ��(��Һ�к���H2O����֬��I2��I)�������ͼһ��ʾ��ʵ����̣�
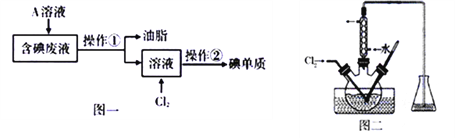
��1��Ϊ�˽������Һ�е�I2��ȫת��ΪI��������ˮ�㣬���Һ�м������Թ�����A��Һ����AӦ�þ���___________�ԡ�
��2������������ƿ�з�Ӧ��ȫ�����Һ���������ڻ�õⵥ�ʣ������ڰ����ಽ�������������Ʒֱ�Ϊ��ȡ��_____��_____���ڲ������б����õ�������ʾ�IJ���������װ�ã���Щ������װ����________________(����)��
��3����������������Һ����ͼ����ʾ��������ƿ�У������������pHԼΪ2���ٻ���ͨ������Cl2��ʹ����30~40�淴Ӧ��д�����з�����Ӧ�����ӷ���ʽ________________��Cl2���ܹ�������Ϊ������Cl2��I2����ΪIO3-��д���÷�Ӧ�����ӷ���ʽ__________��
II.��֬�IJ����Ͷȿ�ͨ����֬���ļӳɷ�Ӧ�ⶨ��ͨ����Ϊ��֬�ĵ�ֵ����ֵԽ����֬�IJ����ͳ̶�Խ�ߡ���ֵ��ָ100g��֬�������յ�I2�Ŀ�������ȡxgij��֬�����뺬ymol I2��Τ����Һ(Τ����Һ�ǵ�ֵ�ⶨʱʹ�õ������Լ�������CH3COOH)�������������I2��cmol/L Na2S2O3����Һ�ζ�(������ָʾ��)������Na2S2O3��ҺV mL(�ζ���ӦΪ:2Na2S2O3+I2��Na2S4O6+2NaI)���ش���������:
��1�������йصζ���˵������ȷ����________(����)��
A����Na2S2O3��ҺӦʢװ�ڼ�ʽ�ζ�����
B���ζ�ʱ�۾�ֻҪע�ӵζ�������Һ����ı仯
C. �ζ��յ�ʱ�����Ӷ��������²ⶨ���ƫ��
D���ζ�����Һ����ɫ����ɫʱӦ������ֹͣ�ζ�
��2���øòⶨ�����ⶨ�ĵ�ֵ��Ҫ����ص�ʵ��У������Ϊ����õĵ�ֵ�ܱ�ʵ�ʵ�ֵ�ͣ�ԭ����_______________________________________________��
��3������֬�ĵ�ֵΪ_____g(��ʽ��ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����л�ѧ���̶�Ӧ�ķ���ʽ��ʾ��ȷ���� ( )
A. ̼��������Һ��ˮ�⣺HCO3-+H2O![]() H3O++CO32-
H3O++CO32-
B. ����ĵ��룺CH3COOH=CH3COO-+H+
C. ̼��Ƶ��ܽ�ƽ��CaCO3(s) ![]() Ca2+(aq)+CO32-(aq)
Ca2+(aq)+CO32-(aq)
D. ������Һ�ʼ��Ե�ԭ��S2-+H2O![]() H2S+2OH-
H2S+2OH-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1�����б�״���������������ʣ���44.8L���飨CH4����6.02��1024��ˮ���Ӣ�196g H2SO4��0.5mol CO2,�����к������������ǣ���д��ţ���ͬ��____��ԭ�������ٵ���____�����������_____��
��2����CO2 ��Na2CO3��Һ ��NaOH ���� ��CaCO3 ��CH3COOH ��NH3��H2O ���Ҵ� ��Һ̬�Ȼ���
���ڵ���ʵ���___________________���ǵ���ʵ���___________________��������ţ�
��3����˫���ŷ���ʾ�����з�Ӧ�ĵ���ת�Ʒ������Ŀ_____________��3S+6KOH![]() K2SO3+2K2S+3H2O
K2SO3+2K2S+3H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��X��Y��Z��M��R Ϊ���ڱ�ǰ������ԭ�������������������Ԫ�أ�X ����������Ԫ�ز�ͬ���ڲ�ͬ���壬Mԭ�������2p�����������δ�ɶԵ��ӣ�Y ����M�γ����ֳ����Ļ����R �����ڱ��ĵ�9�С��ش��������⣺
(1)R ��̬ԭ��M �ܲ�ĵ����Ų�ʽΪ_________________����ԭ�Ӻ����_________�������ĵ��ӡ�
(2)Y��M������X�γ�18e-�ķ��ӣ��������ڼ��Է��ӵ�Ϊ_____________(�ѧʽ)��������Y��M ����ԭ���ӻ���ʽ����Ϊ___________��_________________��
(3)Z ��M���γɵ����ֳ���������ӵļ��ι���Ϊ__________��____________��
(4)R���γ�ԭ�Ӵػ�����R2(CO)8����е�Ϊ52�棬������ˮ���������Ҵ�����������̼�ȣ���һ����Ҫ���л��ϳɴ�������ṹ��ͼ����û���������_________���壬�þ����д��ڵ���������___________(����)��
a.���Ӽ� b.��λ�� C�Ҽ� d.�м� e.��� f.���»���
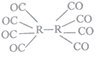
(5)����R�ľ��������֣�417�����µľ����ṹ��ͼ1��417�����ϵľ����ṹ��ͼ2��������λ����_____________����ǰ�߾�������Ϊ��pm��c pm������R��Ħ������ΪMg��mol-1�������ӵ�����ΪNA����ǰ�߾����ܶ�p=_________g��cm-3(�г�����ʽ����)��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵������ȷ����
A.�������Ըı仯ѧ��Ӧ����
B.2 mol SO2��l mol O2���һ��������2 mol SO3
C.ʳ����ڱ����л����ʳ����ʵ�����
D.��ѧ��Ӧ�ﵽ��Ӧ��ʱ������Ӧ���������淴Ӧ���������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com