
X°¢Y°¢Z°¢W «‘™Àÿ÷Ð∆⁄±Ì«∞Àƒ÷Ð∆⁄÷–≥£º˚µƒ‘™Àÿ£¨∆‰œýπÿ–≈œ¢º˚œ¬±Ì£∫
| ‘™Àÿ | œýπÿ–≈œ¢ |
| X | Xµƒª˘Ã¨‘≠◊”∫ÀÕ‚µƒ»˝∏ˆƒÐº∂…œµÁ◊” ˝œýµ» |
| Y | Y”ÎXÕ¨÷Ð∆⁄£¨Yª˘Ã¨‘≠◊”pƒÐº∂µƒ≥…∂‘µÁ◊” ˝”ÎŒ¥≥…∂‘µÁ◊” ˝œýµ» |
| Z | Zµƒµ•÷ «“ª÷÷“¯∞◊…´ªÓ∆√Ω Ù£¨‘⁄ø’∆¯÷–»º…’∫Û…˙≥…µ≠ª∆…´µƒπÃà|
| W | œÚ∫¨W2+µƒ»Ð“∫÷–µŒº”«øºÓ£¨∆‰∞◊…´«‚—ıªØŒÔ‘⁄ø’∆¯÷–—∏ÀŸ±‰≥…ª“¬Ã…´£¨◊Ó∫Û±‰≥…∫Ï∫÷…´ |
£®1£©Àƒ°¢ ¢¯°¢ 2
£®2£©’˝Àƒ√Ê𢠿Î◊”º¸ £®∑«º´–‘£©π≤º€º¸
£®3£©6Na+6H2O+2Fe3+=6Na++3H2°¸+2Fe(OH)3°˝
£®4£©¶§H= +421.69kJ/mol.
Ω‚Œˆ ‘Â∑÷Œˆ£∫∏˘æðƒøÃ·π©µƒ–≈œ¢ø…÷™£∫XŒ™C£ªYŒ™O£ªZŒ™Na;W «Fe°££®1£©26∫≈‘™ÀÿFe‘⁄‘™Àÿ÷Ð∆⁄±Ì÷–Œª”⁄µ⁄Àƒ÷Ð∆⁄µ⁄¢¯◊°£ª˘Ã¨‘≠◊”◊ÓÕ‚≤„”–2∏ˆµÁ◊”°££®2£©Cµƒ◊ÓºÚµ•¬»ªØŒÔ∑÷◊”CCl4≥ ’˝Àƒ√ÊÃÂΩ·ππ°£Na‘⁄ø’∆¯÷–»º…’≤˙…˙Na2O2. ‘⁄¿Î◊”ªØ∫œŒÔNa2O2÷–∫¨”–Na°¢O÷ƺ‰µƒ¿Î◊”º¸º∞O°¢O÷ƺ‰µƒ∑«º´–‘π≤º€º¸°££®3£©NaÕ∂»ÎµΩFeCl3»Ð“∫÷– ±£¨ ◊œ»∑¢…˙∑¥”¶£∫2Na+2H2O=2NaOH+H2°¸£¨»ª∫Û∑¢…˙£∫FeCl3+3NaOH=Fe(OH)3°˝+3NaCl.◊Ð∑Ω≥Ã ΩŒ™6Na+6H2O+2FeCl3=6NaCl+3H2°¸+2Fe(OH)3°˝.¿Î◊”∑Ω≥Ã ΩŒ™£∫6Na+6H2O+2Fe3+=6Na++3H2°¸+2Fe(OH)3°˝ °££®¢Ÿ+¢⁄°¡2£©°¬3’˚¿Ìø…µ√Fe2O3 (s)£´CO(g) =" 2" FeO (s)£´CO2(g) ¶§H= +421.69kJ/mol.
øºµ„£∫øº≤È‘™ÀÿµƒŒª÷√°¢‘≠◊”Ω·ππ°¢∑÷◊”µƒø’º‰ππ–Õ°¢ªØ—ߺ¸µƒ¿ý–Õ°¢¿Î◊”∑Ω≥Ã Ω°¢»»ªØ—ß∑Ω≥Ã Ωµƒ È–¥°£


 ∂·π⁄—µ¡∑µ•‘™∆⁄ƒ©≥Â¥Ã100∑÷œµ¡–¥∞∏
∂·π⁄—µ¡∑µ•‘™∆⁄ƒ©≥Â¥Ã100∑÷œµ¡–¥∞∏ –¬ÀºŒ¨–°π⁄æ¸100∑÷◊˜“µ±æœµ¡–¥∞∏
–¬ÀºŒ¨–°π⁄æ¸100∑÷◊˜“µ±æœµ¡–¥∞∏
| ƒÍº∂ | ∏þ÷–øŒ≥à | ƒÍº∂ | ≥ı÷–øŒ≥à |
| ∏þ“ª | ∏þ“ª√‚∑—øŒ≥ÃÕ∆ºˆ£° | ≥ı“ª | ≥ı“ª√‚∑—øŒ≥ÃÕ∆ºˆ£° |
| ∏þ∂˛ | ∏þ∂˛√‚∑—øŒ≥ÃÕ∆ºˆ£° | ≥ı∂˛ | ≥ı∂˛√‚∑—øŒ≥ÃÕ∆ºˆ£° |
| ∏þ»˝ | ∏þ»˝√‚∑—øŒ≥ÃÕ∆ºˆ£° | ≥ı»˝ | ≥ı»˝√‚∑—øŒ≥ÃÕ∆ºˆ£° |
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫ –գ∫ÃÓø’Â
µ™ø…“‘–Œ≥…∂ý÷÷¿Î◊”£¨»ÁN3-°¢N3£≠°¢NH2£≠°¢NH4+°¢N2H5+°¢N2H62+µ»£¨“—÷™N2H5+”ÎN2H62+ «”…÷––‘∑÷◊”XΩ·∫œH+–Œ≥…µƒ£¨”–¿ýÀ∆”⁄NH4+µƒ–‘÷ °£
¢≈ 1∏ˆN3£≠¿Î◊”∫¨”– ∏ˆµÁ◊”£ª
¢∆ –Œ≥…N2H5+¿Î◊”µƒ÷––‘∑÷◊”Xµƒ∑÷◊” Ω « £ª
X‘⁄—ı∆¯÷–ø…“‘»º…’£¨–¥≥ˆ»º…’∑¥”¶µƒªØ—ß∑Ω≥Ã Ω £ª
¢« –¥≥ˆN2H62+¿Î◊”‘⁄«øºÓ»Ð“∫÷–∑¥”¶µƒ¿Î◊”∑Ω≥Ã Ω °£
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫ –գ∫ÃÓø’Â
Àƒ÷÷∂Ã÷Ð∆⁄‘™Àÿ‘⁄÷Ð∆⁄±Ì÷–µƒŒª÷√»ÁÕº£¨∆‰÷–÷ª”–MŒ™Ω Ù‘™Àÿ°£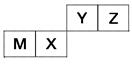
«Îªÿ¥œ¬¡–Œ £∫
(1)’‚–©‘™Àÿµƒ«‚ªØŒÔ÷–£¨ÀƻГ∫ºÓ–‘◊Ó«øµƒ «________(–¥ªØ—ß Ω)£¨∏√«‚ªØŒÔµƒµÁ◊” ΩŒ™________°£
(2)‘™ÀÿX∫Õ‘™ÀÿYµƒ◊Ó∏þº€—ıªØŒÔ∂‘”¶Àƪ،ԵƒÀ·–‘Ωœ»ıµƒ «________£¨¡–染ª∏ˆªØ—ß∑Ω≥à Ω÷§√˜________________________°£
(3)‘™ÀÿZ”Α™ÀÿM◊È≥…µƒªØ∫œŒÔ÷–À˘∫¨ªØ—ߺ¸¿ý–ÕŒ™________£¨‘™ÀÿZ”Α™ÀÿX◊È≥…µƒªØ∫œŒÔ÷–À˘∫¨ªØ—ߺ¸¿ý–ÕŒ™________°£
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫ –գ∫ÃÓø’Â
“—÷™86Rn£®Î±£©ª˘Ã¨‘≠◊”µƒµÁ◊”≈≈≤º ΩŒ™1s22s22p63s23p63d104s24p64d104f145s25p65d106s26p6£¨88Raª˘Ã¨‘≠◊”µƒµÁ◊”≈≈≤º Ωø…ºÚªØŒ™[Rn]7s2°£114∫≈‘™Àÿ «ªØ—ߺ“∫ՌԿ̗ߺ“∫Ð∏––À»§µƒ…–Œ¥∑¢œ÷µƒ‘™Àÿ°£
£®1£©”√ºÚªØµƒ–Œ Ω–¥≥ˆ114∫≈‘™Àÿª˘Ã¨‘≠◊”µƒµÁ◊”≈≈≤º Ω£∫ ______________
________________________________________________________________°£
£®2£©∏˘æð‘≠◊”∫ÀÕ‚µÁ◊”≈≈≤ºµƒÃÿ’˜£¨≈–∂œ114∫≈‘™Àÿ‘⁄÷Ð∆⁄±Ì÷–µƒµ⁄________÷Ð∆⁄________◊°£
£®3£©∏˘æð114∫≈‘™Àÿ‘⁄÷Ð∆⁄±Ì÷–µƒŒª÷√≈–∂œ£¨À¸◊Ó≤ª”¶æþ”–µƒ–‘÷ «________°£
µ⁄“ªµÁ¿ÎƒÐ¥Û”⁄88Ra
¢⁄±´¡÷µÁ∏∫–‘¥Û”⁄3.0
¢€◊Ó∏þªØ∫œº€Œ™£´4º€£¨“≤ø…”–£´2º€
¢Ð◊Ó∏þº€—ıªØŒÔµƒ∂‘”¶Àƪ،Ԝ‘ºÓ–‘
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫ –գ∫ÃÓø’Â
œ¬±Ì «‘™Àÿ÷Ð∆⁄±Ìµƒ“ª≤ø∑÷, ’Î∂‘±Ì÷–µƒ¢Ÿ°´¢‚÷÷‘™Àÿ,ÃÓ–¥œ¬¡–ø’∞◊:
| ÷˜◊ ÷Ð∆⁄ | ¢ÒA | ¢ÚA | ¢ÛA | ¢ÙA | ¢ıA | ¢ˆA | ¢˜A | 0◊ |
| 1 | ¢Ÿ | | | |||||
| 2 | | | | ¢⁄ | ¢€ | ¢Ð | | |
| 3 | ¢ð | | ¢Þ | | ¢þ | | ¢ý | ¢· |
| 4 | ¢‚ | | | | | | | |
| µÁ¿ÎƒÐI(eV) | A | B | C | D | E |
| I1 | 11£Æ3 | 13£Æ6 | 5£Æ2 | 7£Æ6 | 6£Æ0 |
| I2 | 24£Æ4 | 35£Æ1 | 49£Æ3 | 15£Æ0 | 18£Æ8 |
| I3 | 47£Æ9 | 54£Æ9 | 71£Æ6 | 80£Æ1 | 28£Æ4 |
| I4 | 64£Æ5 | 77£Æ4 | 98£Æ9 | 109£Æ2 | 112£Æ0 |
| I5 | 392£Æ1 | 113£Æ9 | 138£Æ3 | 141£Æ3 | 153£Æ7 |
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫ –գ∫ÃÓø’Â
(1)X‘™Àÿµƒ‘≠◊”∫ÀÕ‚”–2∏ˆµÁ◊”≤„£¨∆‰÷–L≤„”–5∏ˆµÁ◊”£¨∏√‘™Àÿ‘⁄÷Ð∆⁄±Ì÷–µƒŒª÷√Œ™________£¨◊Ó∏þº€—ıªØŒÔµƒªØ—ß ΩŒ™________£¨∏√‘™Àÿµƒ«‚ªØŒÔ∫Õ◊Ó∏þº€—ıªØŒÔ∂‘”¶Àƪ،Ô∑¥”¶µƒªØ—ß∑Ω≥Ã ΩŒ™______________________
(2)YŒ™∂Ã÷Ð∆⁄‘™Àÿ£¨»Ù∆‰◊Ó∏þº€—ıªØŒÔ∂‘”¶Àƪ،ԵƒªØ—ß ΩŒ™HYO3£¨‘Ú¥À ±Y‘™ÀÿµƒªØ∫œº€Œ™________£¨Y‘≠◊”µƒ◊ÓÕ‚≤„µÁ◊” ˝Œ™________£¨∆‰∆¯Ã¨«‚ªØŒÔµƒªØ—ß ΩŒ™__________________°£
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫ –գ∫ÃÓø’Â
X°¢Y°¢Z»˝÷÷÷˜◊‘™Àÿµƒµ•÷ ‘⁄≥£Œ¬œ¬∂º «≥£º˚µƒŒÞ…´∆¯Ã£¨‘⁄ µ±Ãıº˛œ¬£¨»˝’þ÷ƺ‰ø…“‘¡Ω¡Ω∑¢…˙∑¥”¶…˙≥…∑÷± «À´∫À°¢»˝∫À∫ÕÀƒ∫Àµƒº◊°¢““°¢±˚»˝÷÷∑÷◊”£¨«“““°¢±˚∑÷◊”÷–∫¨”–X‘™Àÿµƒ‘≠◊”∏ˆ ˝±»Œ™2°√3°£«Îªÿ¥œ¬¡–Œ £∫
(1)‘™ÀÿXµƒ√˚≥∆ «________£¨±˚∑÷◊”µƒµÁ◊” ΩŒ™________°£
(2)»Ùº◊”ÎYµ•÷ ‘⁄≥£Œ¬œ¬ªÏ∫œæÕ”–√˜œ‘œ÷œÛ£¨‘Úº◊µƒªØ—ß ΩŒ™________°£±˚‘⁄“ª∂®Ãıº˛œ¬◊™ªØŒ™º◊∫Õ““µƒ∑¥”¶∑Ω≥Ã ΩŒ™___________________________°£
(3)ªØ∫œŒÔ∂°∫¨X°¢Y°¢Z»˝÷÷‘™Àÿ£¨∂° «“ª÷÷≥£º˚µƒ«øÀ·£¨Ω´∂°”α˚∞¥ŒÔ÷ µƒ¡ø÷Ʊ»1°√1ªÏ∫œ∫ÛÀ˘µ√ŒÔ÷ ŒÏµƒæßÃÂΩ·ππ÷–∫¨”–µƒªØ—ߺ¸Œ™________(—°ÃÓ–Ú∫≈)°£
a£Æ÷ª∫¨π≤º€º¸ b£Æ÷ª∫¨¿Î◊”º¸ c£Æº»∫¨¿Î◊”º¸£¨”÷∫¨π≤º€º¸
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫ –գ∫ÃÓø’Â
œ¬±Ì «‘™Àÿ÷Ð∆⁄±Ìµƒ“ª≤ø∑÷£¨’Î∂‘±Ì÷–µƒ¢Ÿ°´¢·÷÷‘™Àÿ£¨ÃÓ–¥œ¬¡–ø’∞◊£∫
| ÷˜◊ ÷Ð∆⁄°°°° | ¢ÒA | ¢ÚA | ¢ÛA | ¢ÙA | ¢ıA | ¢ˆA | ¢˜A | 0◊ |
| 2 | | | | ¢Ÿ | ¢⁄ | ¢€ | | |
| 3 | ¢Ð | | ¢ð | | | ¢Þ | ¢þ | ¢ý |
| 4 | ¢· | | | | | | | |
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫ –գ∫ÃÓø’Â
≤ø∑÷÷–—ߪؗß≥£º˚‘™Àÿ‘≠◊”Ω·ππº∞–‘÷ »Áœ¬±ÌÀ˘ æ£∫
| –Ú∫≈ | ‘™Àÿ | Ω·ππº∞–‘÷ |
| ¢Ÿ | A | Aµ•÷ «…˙ªÓ÷–µƒ≥£º˚Ω Ù£¨À¸”–¡Ω÷÷¬»ªØŒÔ£¨œý∂‘∑÷◊”÷ ¡øœý≤Ó35.5 |
| ¢⁄ | B | B‘≠◊”◊ÓÕ‚≤„µÁ◊” ˝ «ƒ⁄≤„µÁ◊”◊Ð ˝µƒ1/5 |
| ¢€ | C | C «≥£º˚ªØ∑ µƒ÷˜“™‘™Àÿ£¨µ•÷ ≥£Œ¬œ¬≥ ∆¯Ã¨ |
| ¢Ð | D | Dµ•÷ ±ª”˛Œ™°∞–≈œ¢∏Ô√¸µƒ¥þªØº¡°±£¨ «≥£”√µƒ∞εºÃÂ≤ƒ¡œ |
| ¢ð | E | Õ®≥£«Èøˆœ¬£¨E√ª”–’˝ªØ∫œº€£¨A°¢B°¢C°¢D°¢F∂ºƒÐ”ÎE–Œ≥…ªØ∫œŒÔ |
| ¢Þ | F | F‘⁄÷Ð∆⁄±Ì÷–ø…“‘≈≈‘⁄¢ÒA◊£¨“≤”–»À÷≥ˆ≈≈‘⁄¢˜A◊ |
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
∞Ÿ∂»÷¬–≈ - ¡∑œ∞≤·¡–±Ì - ‘¡–±Ì
∫˛±± °ª•¡™Õ¯Œ•∑®∫Õ≤ª¡º–≈œ¢æŸ±®∆Ωî | Õ¯…œ”–∫¶–≈œ¢æŸ±®◊®«¯ | µÁ–≈’©∆≠柱®◊®«¯ | …Ê¿˙ ∑–ÈŒÞ÷˜“”–∫¶–≈œ¢æŸ±®◊®«¯ | …Ê∆Û«÷»®æŸ±®◊®«¯
Œ•∑®∫Õ≤ª¡º–≈œ¢æŸ±®µÁª∞£∫027-86699610 柱®” œ‰£∫58377363@163.com