
���ξۺ���SPLA�ɾ����з�Ӧ·�ߵõ�(���ַ�Ӧ����δע��)��

��֪��SPLA�Ľṹ��ʽΪ
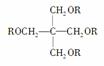 ������RΪ
������RΪ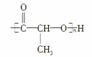
(1)������________��(������ࡱ)��A��������____________��
(2)��ȩ�ɲ��������Ʊ��ķ���֮һ��
________________________________________________________________________
________________________________________________________________________(�û�ѧ����ʽ��ʾ���ɶಽ)��
(3)D��E�Ļ�ѧ��Ӧ��������________��Ӧ��D�ṹ����3����ͬ�Ļ��ţ���1 mol D����2 mol Ag(NH3)2OH��Ӧ����D�Ľṹ��ʽ��____________��D��������Һ��Ӧ�Ļ�ѧ����ʽΪ________________________________________________________________________
________________________________________________________________________��
(4)B��ֱ��ͬ���칹��G�ķ����в�������G�Ȳ�����NaHCO3��Һ��Ӧ���ֲ���������Cu(OH)2����Һ��Ӧ����1 mol G������Na��Ӧ����1 mol H2����G�Ľṹ��ʽΪ________________________________________________________________________��
(5)B�ж�����ˮ����������ֲ���Ľṹ��ʽΪ____________________��
____________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��0.2 mol��L��1 HA��Һ��0.2 mol��L��1 NaOH��Һ�������ϣ���û����Һ��c(Na��)>c(A��)����(�á�>������<��������д���пհ�)��
(1)�����Һ��c(HA)________c(A��)��
(2)�����Һ��c(HA)��c(A��)________0.1 mol��L��1��
(3)�����Һ����ˮ�������c(OH��)______0.2 mol��L��1 HA��Һ����ˮ�������c(H��)��
(4)25��ʱ�����ȡ0.2 mol��L��1 HA��Һ��0.1 mol��L��1 NaOH��Һ�������ϣ���û����Һ��pH<7����HA�ĵ���̶�________NaA��ˮ��̶ȡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�¶������ݻ�Ϊ 2L ���ܱ������У�������Ӧ 2X(g) + Y(g)  2W(g)����H��0�������� 2 mol X �� 1 mol Y����20 s�ﵽƽ��ʱ������ 0.4 molW������˵����ȷ���ǣ���
2W(g)����H��0�������� 2 mol X �� 1 mol Y����20 s�ﵽƽ��ʱ������ 0.4 molW������˵����ȷ���ǣ���
�������¶ȣ�W �����������С����H ����
���� Y ��Ũ�ȱ仯��ʾ�ķ�Ӧ����Ϊ 0.01 mol/(L·s)
���������������������£����� 1 mol X ���� X �� Y ��ת���ʾ����
������ѹǿ������Ӧ���������淴Ӧ���ʼ�С����ƽ��������Ӧ�����ƶ�
������������ͨ�� 2 mol X �� 1 mol Y���ﵽƽ��ʱ��X��Y ��ת���ʾ�����
A���٢� B���� C���ڢۢ� D���ܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��ʾ���Թ���ʢ�н�Ũ�İ�ˮ����ˮ������з�̪����dz��ɫ��

(1)���Թ�����ˮԡ�ķ�ʽ���ȣ��۲��Թ��е���Һ�������____________(�������д����������ݣ��������д���������ԭ��)��
(2)�Ѽ��Ⱥ���Թܷ���Լ20���ˮ����ȴ���۲��Թ��е���Һ���Ƿ������Ե���������________________________________________________________________________
(�������д����������ݣ��������д���������ԭ��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ʳƷ�깺������������Һ����������Ӧʵ�飬�����ܵõ�������������һ�����ԭ����(����)
A�����DZ����л�ԭ��
B�����DZ���ԭ
C��ʵ����������Ƿ���ˮ��
D������������������������в���ˮ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵���в���ȷ����(����)
A����ɵ����ʵİ����Ἰ�����Ǧ�������
B�������������Է�����ᴿ������
C��DNA���������Ŵ���Ϣ�����塢�����ʺϳɵ�ģ��
D��RNA��Ҫ������ϸ�����У�������DNA�ṩ����Ϣ�������ڵ����ʵĺϳ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ҩ�����ǽ�����C��H��O��N 4��Ԫ�ص��л����������Է�������195<Mr<200������O����������Ϊ32.49%��N����������Ϊ7.11%�����ⶨ��ҩ���;����������ʺͽṹ������
��.��FeCl3��Һ����ɫ��
��.1 mol��ͺͺ�1 mol HCl��������Һ���ߺͺ�3 mol NaOH��NaOH��Һ����ǡ����ȫ��Ӧ��
��.��ͷ����к���1����������������2��������λ����ͬȡ����A����1������A������λ��ȡ������
��.2���Ӷ����ȥ2����ˮ�����γɺ���3��6Ԫ�����л��
�ش��������⣺
(1)��͵�Ħ������Ϊ________��
(2)��͵Ľṹ��ʽΪ
________________________________________________________________________��
(3)2���Ӷ����ȥ2����ˮ�����γɵĺ���3��6Ԫ�����л���Ľṹ��ʽΪ
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
1 000 Kʱ��ӦC(s)��2H2(g)
 CH4(g)��K��8.28��107 (mol��L��1)��1�������������ʵ���Ũ�ȷֱ�ΪH2 0.7 mol��L��1��CH4 0.2 mol��L��1ʱ��������Ӧ(����)
CH4(g)��K��8.28��107 (mol��L��1)��1�������������ʵ���Ũ�ȷֱ�ΪH2 0.7 mol��L��1��CH4 0.2 mol��L��1ʱ��������Ӧ(����)
A�������ƶ� B�������ƶ� C���ﵽƽ�� D����һ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������������������м�����Ҫ�����ã��������й��ס���Ч���Ͷ���ɱ�������������ǽ��չ�����ơ�
(1)Cl2��H2O2��ClO2(��ԭ����ΪCl��)��O3(1 mol O3ת��Ϊ1 mol O2��1 mol H2O)�����ʳ��������������������ʵ�����������������Ч����ߵ���________ (�����)��
A��Cl2 B��H2O2
C��ClO2 D��O3
(2)H2O2��ʱ����Ϊ��ҵ��Һ���������С���ɫ�������������ơ��������ɿ�ҵ��Һ�е��軯��(��KCN)�������·�Ӧʵ�֣�KCN��H2O��H2O2===A��NH3������������A�Ļ�ѧʽΪ________��H2O2����Ϊ����ɫ����������������_________________________________________��
(3)Ư����������(NaClO2)�ڳ����¡��ڰ����ɱ���һ�ꡣ������ȶ��ɷֽ⣬��Ӧ�����ӷ���ʽΪHClO2�D��ClO2����H����Cl����H2O(δ��ƽ)���ڸ÷�Ӧ�У�����1 mol ClO2����ʱת�Ƶĵ�����ԼΪ________��
(4)��84������Һ(��Ҫ�ɷ���NaClO)�ͽ��(��Ҫ�ɷ���Ũ����)���ܻ��ã�ԭ����__________________________________(�����ӷ���ʽ��ʾ)�������ȼҵ�IJ������������84������Һ��д���йط�Ӧ�Ļ�ѧ����ʽ��______________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com