
��һ���������İ�������粒����������Ƶ��ܱ���������У��������������������������������Բ��ƣ����ں㶨�¶���ʹ��ﵽ�ֽ�ƽ�⣺NH2COONH4��s��  2NH3��g����CO2��g��
2NH3��g����CO2��g��
ʵ���ò�ͬ�¶��µ�ƽ�����������±���
�¶�/�� | 15.0 | 20.0 | 25.0 | 30.0 | 35.0 |
ƽ����ѹ ǿ/kPa | 5.7 | 8.3 | 12.0 | 17.1 | 24.0 |
ƽ��������Ũ��/mol�� L��1 | 2.4�� 10��3 | 3.4�� 10��3 | 4.8�� 10��3 | 6.8�� 10��3 | 9.4�� 10��3 |
��1�������жϸ÷ֽⷴӦ�Ѿ��ﵽƽ�����________��
A��2v��NH3����v��CO2��
B���ܱ���������ѹǿ����
C���ܱ������л��������ܶȲ���
D���ܱ������а����������������
��2�����ݱ�����������ʽ����25.0 ��ʱ�ķֽⷴӦƽ�ⳣ����_______________��
��3��ȡһ�����İ�������粒������һ�����������ܱ��������������25.0 ���´ﵽ�ֽ�ƽ�⡣���ں�����ѹ�������������������粒����������________������������������������������������
��1��BC
��2��K��c2��NH3����c��CO2���� ��
�� ��
�� ����4.8��10��3��3��1.6��10��8
����4.8��10��3��3��1.6��10��8
��3������
����������1��A��ܱ�ʾ�����淴Ӧ������ȣ�B����������Ӧ����������������������ܱ�������ѹǿ��������Ӧ�ﵽƽ�⣻C���������ƽ�ⷢ���ƶ������������ܶȷ����ı䣻D�Ӧ���ǹ�����NH3���������ʼ��Ϊ ��
��
��2���轫25 ������Ũ��ת��ΪNH3��CO2��Ũ�ȣ�
c��NH3���� ��4.8��10��3 mol��L��1��3.2��10��3 mol��L��1��c��CO2����
��4.8��10��3 mol��L��1��3.2��10��3 mol��L��1��c��CO2���� ��4.8��10��3 mol��L��1��1.6��10��3 mol��L��1��
��4.8��10��3 mol��L��1��1.6��10��3 mol��L��1��
K����3.2��10��3��2��1.6��10��3��1.6��10��8��
��3������ѹǿ��ƽ�����淴Ӧ�����ƶ���������������


 ��ʦ����ָ���ο�ʱϵ�д�
��ʦ����ָ���ο�ʱϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2014��߶��˽̻�ѧѡ����2-1-2֬����Ȳ��֬������Դ��Ӧ����ϰ���������棩 ���ͣ������
��֪�������ʣ�

�밴Ҫ����գ�
(1)д���٢�������________��________��
(2)д���������ķ���ʽ________��________��
(3)��Ϊͬ���칹�����________��
(4)д����������ʵ�����Br2��Ӧ�Ļ�ѧ����ʽ��_______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014����л�ѧ���ִ���ѵ����ר��1������ɷ������ʼ���ѧ������ϰ���������棩 ���ͣ�ѡ����
��H2O�ĵ���ƽ�ⲻ����Ӱ������ǣ� ����
A��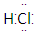 B��26M3�� C.
B��26M3�� C. D��
D��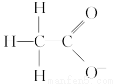
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014����л�ѧ���ִ���ѵ���� ר��7�������Һ��ϰ���������棩 ���ͣ������
�����г������ؽ�����Ⱦ���У�����Ǧ���̡������ӡ�������ҵ��ˮ�к��е�Cr2O72-��CrO42-�����õķ��������֡�
����1����ԭ������
�÷��Ĺ�������Ϊ ��
��
���е���������ƽ��2CrO42-����ɫ����2H�� Cr2O72-����ɫ����H2O��
Cr2O72-����ɫ����H2O��
��1��д����������Ӧ��ƽ�ⳣ������ʽ_________________________________��
��2�����ڵ�������Ӧ������˵����ȷ����________��
A��ͨ���ⶨ��Һ��pH�����жϷ�Ӧ�Ƿ��Ѵ�ƽ��״̬
B���÷�ӦΪ������ԭ��Ӧ
C��ǿ���Ի�������Һ����ɫΪ��ɫ
��3��������������ԭ0.1 mol Cr2O72-����Ҫ________mol��FeSO4��7H2O��
��4��������������Cr��OH��3�������������ɵij���Ϊ________������Һ�д������³����ܽ�ƽ�⣺Cr��OH��3��s�� Cr3����aq����3OH����aq������������Cr��OH��3���ܶȻ�Ksp��10��32����c��Cr3��������10��5 mol��L��1ʱ����Ϊc��Cr3�����Ѿ���ȫ�������ֽ���������Һ��pH����4����ͨ������˵��Cr3���Ƿ������ȫ����д��������̣���____________________________________________________________________________��
Cr3����aq����3OH����aq������������Cr��OH��3���ܶȻ�Ksp��10��32����c��Cr3��������10��5 mol��L��1ʱ����Ϊc��Cr3�����Ѿ���ȫ�������ֽ���������Һ��pH����4����ͨ������˵��Cr3���Ƿ������ȫ����д��������̣���____________________________________________________________________________��
����2����ⷨ
��5��ʵ����������ͼװ��ģ���ⷨ������Cr2O72-�ķ�ˮ�����ʱ������ӦʽΪ________��������ӦʽΪ________���õ��Ľ������������������ɳ�����ȫ����ˮ�ĵ���ƽ��ǶȽ�����ԭ����___________________________________________________________��
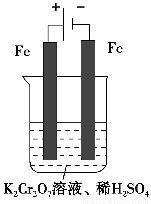
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014����л�ѧ���ִ���ѵ���� ר��7�������Һ��ϰ���������棩 ���ͣ�ѡ����
����Һ�������25 �棬�й�������ȷ���� ����������
A��ij������Һ��pH��7���������һ���Ǽ��ǿ��������
B��pH��6.5��ţ����c��H������pH��4.5��H2SO4��Һ��c��H������100��
C��pH��3�Ĵ�����pH��11��NaOH��Һ�������Ϻ���Һ�У�c��CH3COO������c��Na������c��H������c��OH����
D��AgCl�ڵ�Ũ�ȵ�CaCl2��Һ��NaCl��Һ�е��ܽ����ͬ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014����л�ѧ���ִ���ѵ���� ר��6��ѧ��Ӧ���ʺͻ�ѧƽ����ϰ���������棩 ���ͣ�ѡ����
��KI��Һ�д�������ƽ�⣺I2��aq����I����aq��=I3-��aq������ò�ͬ�¶��¸÷�Ӧ��ƽ�ⳣ��K�����ʾ��
t/�� | 5 | 15 | 25 | 35 | 50 |
K | 1 100 | 841 | 689 | 533 | 409 |
����˵����ȷ���� ����������
A����ӦI2��aq����I����aq��  I3-��aq������H>0
I3-��aq������H>0
B���������������������¶�����Һ��c��I3-����С
C���÷�Ӧ��ƽ�ⳣ������ʽΪK��
D��25 ��ʱ������Һ�м�������KI������ƽ�ⳣ��KС��689
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014����л�ѧ���ִ���ѵ���� ר��5��ѧ��Ӧ�������仯��ϰ���������棩 ���ͣ������
��1������ʯ[��Ҫ�ɷ�Ca5��PO4��3F]�ڸ������Ʊ����ף�P4�����Ȼ�ѧ����ʽΪ��4Ca5��PO4��3F��s����21SiO2��s����30C��s��=3P4��g����20CaSiO3��s����30CO��g����SiF4��g������H
��������Ӧ�������������������________��
����֪��ͬ�����£�
4Ca5��PO4��3F��s����3SiO2��s��=6Ca3��PO4��2��s����2CaSiO3��s����SiF4��g������H1
2Ca3��PO4��2��s����10C��s��=P4��g����6CaO��s����10CO��g������H2
SiO2��s����CaO��s��=CaSiO3��s������H3
����H1����H2����H3��ʾ��H����H��____________��
��2�������գ���H2O2��H2SO4�Ļ����Һ���ܳ�ӡˢ��·�������ĩ�е�ͭ����֪��
��Cu��s����2H����aq��=Cu2����aq����H2��g����H1����64.39 kJ��mol��1
��2H2O2��l��=2H2O��l����O2��g����H2����196.46 kJ��mol��1
��H2��g����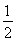 O2��g��=H2O��l����H3����285.84 kJ��mol��1
O2��g��=H2O��l����H3����285.84 kJ��mol��1
��H2SO4��Һ����Cu��H2O2��Ӧ����Cu2����H2O���Ȼ�ѧ����ʽΪ_________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014����л�ѧ���ִ���ѵ���� ר��4���ʽṹ��Ԫ����������ϰ���������棩 ���ͣ�ѡ����
���й���ָ�����ӹ��ɵ�������������ȷ���� ����������
A��37Cl��39K������ͬ��������
B����114��Ԫ�ص�һ�ֺ���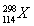 ��
��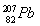 ������ͬ������������
������ͬ������������
C��H3O����OH��������ͬ���������͵�����
D��O22-��S2��������ͬ���������͵�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014����л�ѧ���ִ���ѵ���� ר��13��ѧʵ���ۺ�Ӧ����ϰ���������棩 ���ͣ������
�������ȣ�ClO2����Ŀǰ�����Ϲ��ϵĵ��Ĵ���Ч����������������һ�ֻ���ɫ�����壬������ˮ��
��.��1��ClO2����KClO3��H2SO4���ڵ���������Na2SO3��Ӧ�Ƶá���÷�Ӧ�����������뻹ԭ��������ʵ���֮����________��
��.ʵ����Ҳ����NH4Cl�����ᡢNaClO2���������ƣ�Ϊԭ���Ʊ�ClO2�����������£�

��2��д�����ʱ������Ӧ�Ļ�ѧ����ʽ��________________________________��
��3����ȥClO2�е�NH3��ѡ�õ��Լ���________��������ţ�
A������ʳ��ˮ B����ʯ��
C��Ũ���� D��ˮ
��4���ⶨClO2����ͼ���Ĺ������£�����ƿ�м��������ĵ⻯������100 mLˮ�ܽ�����ټ�3 mL������Һ���ڲ���Һ����м���ˮ�������ɵ�ClO2����ͨ����������ƿ�б����գ�����������е�ˮ��Һ������ƿ�У����뼸�ε�����Һ����c mol��L��1��������Ʊ���Һ�ζ���I2��2S2O32-=2I����S4O62-��������ȥV mL�����������Һ��

��װ���в���Һ��ܵ�������______________________________________��
����д��������������������⻯����Һ��Ӧ�����ӷ���ʽ__________________________��
���ζ��յ��������_______________________________________________��
�����ͨ��ClO2������m��ClO2����________�����ú�c��V�Ĵ���ʽ��ʾ��
��5����ClO2������������ˮ��pHΪ5.5��6.5��������һ���������岻���������������ClO2-��2001���ҹ��������涨������ˮ��ClO2-����Ӧ������0.2 mg��L��1��������ˮ��ClO2-�ĺ����������������м���������ij��ԭ�����÷�Ӧ������������________���ѧʽ�����䷢����Ӧ�����ӷ���ʽΪ_________________________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com