
�绯ѧԭ���ڹ�ҵ������������Ҫ�����ã���������ѧ֪ʶ�ش��й����⡣
(1)�õ��ķ�����������Һ����Ϊ��������о�������Ҫ��ʵ�����壬������ת��Ϊ�������ǵ�ⷨ�������������һ����Ҫ���ݡ����ǵ������������ʵ��װ�ã�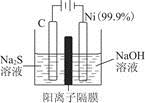
����֪�����ķ�ӦΪ(x��1)S2��=Sx��S2����2xe�����������ĵ缫��Ӧʽ��____________________________
����Ӧת��x mol����ʱ���������������Ϊ____________(��״����)��
�ڽ�Na2S��9H2O����ˮ������������Һʱ��ͨ�����ڵ����������ܽ⡣��ԭ����(�����ӷ�Ӧ����ʽ��ʾ)��___________________________��
(2)MnO2��һ����Ҫ�������ܲ��ϣ��Ʊ�MnO2�ķ���֮һ����ʯīΪ�缫������ữ��MnSO4��Һ�������ĵ缫��ӦʽΪ______________________������Ǧ����Ϊ��Դ����ữ��MnSO4��Һ����ͼ��ʾ��Ǧ���ص��ܷ�Ӧ����ʽΪ_______________________��
����������4 mol H��������ʱ�����·��ͨ���ĵ��ӵ����ʵ���Ϊ________��MnO2�����۲���Ϊ________g��
(3)��ͼ���װ�ÿ��Ƶþ��о�ˮ���õ�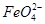 ��ʵ������У������������������Y������Һ������
��ʵ������У������������������Y������Һ������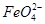

�ٵ������У�X������Һ��pH________(�������С�����䡱)��
�ڵ������У�Y�������ĵ缫��ӦΪFe��6e����8OH��=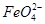 ��4H2O��______________________________������X���ռ���672 mL���壬��Y���ռ���168 mL����(��������Ϊ��״��ʱ�������)����Y�缫(���缫)��������________g��
��4H2O��______________________________������X���ռ���672 mL���壬��Y���ռ���168 mL����(��������Ϊ��״��ʱ�������)����Y�缫(���缫)��������________g��
(1)��2H2O��2e��=2OH����H2��(��2H����2e��=H2��)��11.2xL
��2S2����O2��2H2O=2S����4OH��
(2)Mn2����2e����2H2O=MnO2��4H��
Pb��PbO2��2H2SO4=2PbSO4��2H2O
2 mol��87
(3)����4OH����4e��=2H2O��O2����0.28
����(1)���ʱ��ˮ�����H�������������õ��ӻ�ԭ��Ӧ������H2�����ݵ����غ��֪��x mol����ת�ƣ�����H2 0.5x mol��S2�����н�ǿ��ԭ�ԣ��ױ������е�������������������Һʱ��Ҫ��������������
(2)��������Mn2��ʧ��������MnO2�������е���Ԫ����Դ��ˮ������H�����ٽ��缫����ʽ��ƽ���ɡ�
(3)ͼ��X���ĵ缫��ӦΪ2H����2e��=H2��(��2H2O��2e��=H2����2OH��)������X������pH��������������672 mL����֪�õ�����Ϊ0.06 mol��Y����������Ϊ168 mL��ʧ������0.03 mol���ɵ�ʧ�����غ��֪��ʧ������Ϊ0.03 mol���ɵ缫��Ӧ��֪���ܽ�Ϊ0.005 mol����0.28 g��


 ��У���һ��ͨϵ�д�
��У���һ��ͨϵ�д� �γ̴����Ծ�����100��ϵ�д�
�γ̴����Ծ�����100��ϵ�д� �¾�����ĩ���100��ϵ�д�
�¾�����ĩ���100��ϵ�д� ȫ�ܴ���100��ϵ�д�
ȫ�ܴ���100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(8��)����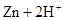 �T�T
�T�T ���ķ�Ӧ���ԭ��ء�
���ķ�Ӧ���ԭ��ء�
��1��ԭ�����ʹ�õĵ������Һ�� ��
��2��д���缫��Ӧʽ��
������ ��
����: ��
��3���ڷ����л�����ԭ��ص�ͼ��������缫���ϵ����ơ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
﮵Ļ�������;�㷺��Li3N�Ƿdz���ǰ;�Ĵ�����ϣ�LiFePO4��Li2FeSiO4�ȿ�����Ϊ��ص��������ϡ��ش��������⣺
��1������ڴ�������ȼ�տ��Ƶ�Li3N���䷴Ӧ�Ļ�ѧ����Ϊ ��
��2��������������м���ʱ�ɵõ�����ﮣ�LiNH2�����䷴Ӧ�Ļ�ѧ����ʽΪ��
Li3N+2H2 LiNH2+2LiH����������Ϊ ���ѧʽ������270��ʱ���÷�Ӧ���������ų�H2���������﮿���Ϊ������ϣ������������ɴ�Li3N������ %����ȷ��0.1����
LiNH2+2LiH����������Ϊ ���ѧʽ������270��ʱ���÷�Ӧ���������ų�H2���������﮿���Ϊ������ϣ������������ɴ�Li3N������ %����ȷ��0.1����
��3����Li2CO3��FeC2O4��2H2O��SiO2��ĩ���Ȼ�ϣ���800���������ս�6Сʱ�Ƶ�Li2FeSiO4��д����Ӧ�Ļ�ѧ����ʽ ���Ʊ�Li2FeSiO4�Ĺ��̱����ڶ��������Χ�н��У���ԭ���� ��
��4����һ��Ũ���������李��Ȼ�﮻����Һ��Ϊ���Һ��������Ϊ������ʯīΪ�������������LiFePO4�����������ĵ缫��ӦʽΪ ��
��5����������﮵�س�ŵ�����У�����LiFePO4��Li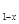 FePO4֮���ת������طŵ�ʱ���������ķ�ӦΪLiXC6��Xe��
FePO4֮���ת������طŵ�ʱ���������ķ�ӦΪLiXC6��Xe�� XLi++6C��д����طŵ�ʱ�ĵ缫��Ӧ�Ļ�ѧ����ʽ ��
XLi++6C��д����طŵ�ʱ�ĵ缫��Ӧ�Ļ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ijʵ��С���ͬѧ������ͼ��ʾװ���������йػ�ѧʵ�飬��������пո�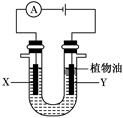
(1)��U�ι���ʢ����������Һ��X��Y�缫�ֱ�Ϊʯī���������������г��ֵ������� ��
U�ι��м��������ֲ���������� ��
(2)���һ��ʱ���ijͬѧ����Դ���ӣ���ʱ���ֵ������� ���йصĻ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������ͼ�ش��������⣺
��A��B��Ϊ̼������������Һ��������
��1����װ���� �أ���װ���� ����Fe��Ϊ ����A��Ϊ ��
��2��A���ĵ缫��Ӧ
��3�����׳���Fe�ܽ�0��3 mol�����ҳ��в���������������״����Ϊ L��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ��ʾ��p��qΪֱ����Դ��������A�ɽ�������X�Ƴɣ�B��C��DΪ���缫����ͨ��Դ������X������B����ͬʱC��D�ϲ������ݣ��Իش�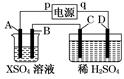
(1)pΪ ����A�������� ��Ӧ��
(2)CΪ �������ռ��� ��DΪ �������ռ��� ��
(3)C���ĵ缫��ӦʽΪ ��
(4)�ڵ������У���C��D�����ϲ�������������ʵ���������±���
| ʱ��(min) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| ������������ �����(cm3) | 6 | 12 | 20 | 29 | 39 | 49 | 59 | 69 | 79 | 89 |
| ������������ �����(cm3) | 2 | 4 | 7 | 11 | 16 | 21 | 26 | 31 | 36 | 41 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����������ض�����Ҫ�Ĺ�ҵ��Ʒ����ش�:
(1)��ҵұ�����Ļ�ѧ����ʽ������������������������
(2)��������������Һ��Ӧ�����ӷ���ʽ��������������������������������
(3)��ҵƷ�������ص���Һ�к���ijЩ����������,�������ӽ���Ĥ������ᴿ��
������װ�������ӽ���Ĥ(ֻ����������ͨ��),�乤��ԭ����ͼ��ʾ��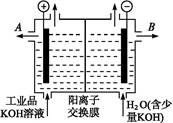
�ٸõ��۵�������Ӧʽ������������������������
��ͨ�翪ʼ��,����������ҺpH������,�����ԭ��������������������������
�۳�ȥ���ʺ������������Һ����Һ������������(��д��A����B��)������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ȼҵ��������Ļ�ѧ��ҵ֮һ������Ĥ��ⷨΪĿǰ�ձ�ʹ�õ�������������������������ͼ��ʾ��
��1���������п���ѭ���������� ��
��2����ⷨ�Ƽ����Ҫԭ���DZ���ʳ��ˮ�����ڴ���ˮ�к���Ca2+��Mg2+��SO42���������ʣ������ڽ������ǰ��Ҫ�������ξ��ƣ�д��һ�ξ����з��������ӷ���ʽ ����ʳ��ˮ���������ξ��ƾ�ֱ�ӽ�������Ĥ���ۻ����ʲô��� ��
��3����ͼ�ǹ�ҵ�ϵ�ⱥ��ʳ��ˮ�����ӽ���Ĥ����ʾ��ͼ(�����ý��������Ƴɣ�������̼�����Ƴ�)����B�������������� ��E�缫�������� ������ܷ�Ӧ�����ӷ���ʽΪ ��
��4���������۳����ĵ���ˮ�У����������������ܽ��ȣ���Ҫ����8����9��������������Һ���䳹�׳�ȥ���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��5����֪�ڵ����У�ÿСʱͨ��1�����ֱ������Բ���1.492g���ռij������300�����۴�������8Сʱ���Ƶ�32%���ռ���Һ���ܶ�Ϊ1.342��/m3��113m3�����۵ĵ���ǿ��1.45 ��104A���õ��۵ĵ��Ч��Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�мס�����λͬѧ��������ԭ��ط�Ӧ�������Ļ��˳�����˾���þƬ����Ƭ���缫������ͬѧ���缫����6 mol��L��1��H2SO4��Һ�У���ͬѧ���缫����6 mol��L��1��NaOH��Һ�У���ͼ��ʾ��
��1��д�����������ĵ缫��Ӧʽ_____________________________��
��2�����и���Ϊ________���ܷ�Ӧ�����ӷ���ʽ��
______________________________________________________��
��3�����������ͬѧ����Ϊ������ԭ��صĵ缫����������ǽ������ɸ������ϵĽ���Ӧ�ȹ����������ϵĽ������á�������жϳ�________��Ը�ǿ�����һ��жϳ�________��Ը�ǿ������дԪ�ط��ţ�
��4���ɴ�ʵ��ó������н����У���ȷ����________��
| A������ԭ��ط�Ӧ�жϽ������˳��ʱӦע��ѡ����ʵĽ��� |
| B��þ�Ľ����Բ�һ�������Ľ�����ǿ |
| C����ʵ��˵���������˳����ѹ�ʱ��û��ʵ�ü�ֵ�� |
| D����ʵ��˵����ѧ�о������ӡ���Ӧ������Ӱ��ϴ����Ӧ�������������� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com