
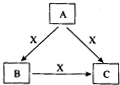
A”¢B”¢C”¢XŹĒ֊ѧ»Æѧ³£¼ūĪļÖŹ£¬¾łÓɶĢÖÜĘŚŌŖĖŲ×é³É£¬×Ŗ»Æ¹ŲĻµČēĶ¼ĖłŹ¾”£
ĒėÕė¶ŌŅŌĻĀ²»Ķ¬Ēéæö»Ų“š£ŗ
£Ø1£©ČōA”¢B”¢CµÄŃęÉ«·“Ó¦¾ł³Ź»ĘÉ«£¬Ė®ČÜŅŗ¾łĪŖ¼īŠŌ”£
¢ŁAÖŠĖłŗ¬ÓŠµÄ»Æѧ¼üŹĒ_____________”£
¢Ś½«4.48 L£Ø±ź×¼×“æöĻĀ£©XĶØČė100mL 3 mol£ÆL AµÄĖ®ČÜŅŗŗó£¬ČÜŅŗÖŠĄė×ÓÅضČÓɓ󵽊”µÄĖ³Šņ ĪŖ_______________________________________”£
ĪŖ_______________________________________”£
¢Ū×ŌČ»½ēÖŠ“ęŌŚB”¢CŗĶH2O°“Ņ»¶Ø±ČĄż½į¾§¶ų³ÉµÄ¹ĢĢå”£Č”Ņ»¶ØĮæøĆ¹ĢĢåČÜÓŚĖ®Åä³É100mLČÜŅŗ£¬²āµĆČÜČÜÖŠ½šŹōŃōĄė×ÓµÄÅضČĪŖ0.5 mol£ÆL”£ČōČ”ĻąĶ¬ÖŹĮæµÄ¹ĢĢå¼ÓČČÖĮŗćÖŲ£¬Ź£Óą¹ĢĢåµÄÖŹĮæĪŖ__________”£
£Ø2£©ČōAĪŖ¹ĢĢ¬·Ē½šŹōµ„ÖŹ£¬AÓėXĶ¬ÖÜĘŚ£¬³£ĪĀ³£Ń¹ĻĀCĪŖ°×É«¹ĢĢ壬B·Ö×ÓÖŠø÷Ō×Ó×īĶā²ć¾łĪŖ8e ½į¹¹”£
½į¹¹”£
¢ŁĻĀĮŠÓŠ¹ŲBĪļÖŹµÄŠšŹöÕżČ·µÄŹĒ
a”¢BµÄ·Ö×ÓŹ½ĪŖAX b”¢BĪŖ¹²¼Ū»ÆŗĻĪļ
c”¢B·Ö×Ó³ŹČż½Ē׶ŠĪ d”¢BŠŌÖŹĪČ¶Ø£¬²»Óė³żXĶāµÄČĪŗĪĪļÖŹ·¢Éś»Æѧ·“Ó¦
¢ŚCÓėĖ®¾ēĮŅ·“Ó¦£¬Éś³ÉĮ½ÖÖ³£¼ūĖį£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
 A”¢B”¢C”¢XŹĒ֊ѧ»Æѧ³£¼ūĪļÖŹ£¬×Ŗ»Æ¹ŲĻµČēĶ¼ĖłŹ¾£®ĒėÕė¶ŌŅŌĻĀ²»Ķ¬Ēéæö»Ų“šĪŹĢā£ŗ
A”¢B”¢C”¢XŹĒ֊ѧ»Æѧ³£¼ūĪļÖŹ£¬×Ŗ»Æ¹ŲĻµČēĶ¼ĖłŹ¾£®ĒėÕė¶ŌŅŌĻĀ²»Ķ¬Ēéæö»Ų“šĪŹĢā£ŗ
| ||
| ||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 A”¢B”¢C”¢XŹĒ֊ѧ»Æѧ³£¼ūĪļÖŹ£¬¾łÓɶĢÖÜĘŚŌŖĖŲ×é³É£¬×Ŗ»Æ¹ŲĻµČēĶ¼ĖłŹ¾£®ĒėÕė¶ŌŅŌĻĀ²»Ķ¬Ēéæö»Ų“š£ŗ
A”¢B”¢C”¢XŹĒ֊ѧ»Æѧ³£¼ūĪļÖŹ£¬¾łÓɶĢÖÜĘŚŌŖĖŲ×é³É£¬×Ŗ»Æ¹ŲĻµČēĶ¼ĖłŹ¾£®ĒėÕė¶ŌŅŌĻĀ²»Ķ¬Ēéæö»Ų“š£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
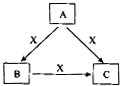 A”¢B”¢C”¢XŹĒ֊ѧ»Æѧ³£¼ūĪļÖŹ£¬¾łÓɶĢÖÜĘŚŌŖĖŲ×é³É£¬×Ŗ»Æ¹ŲĻµČēĶ¼ĖłŹ¾£®ĒėÕė¶ŌŅŌĻĀČżÖÖ²»Ķ¬Ēéæö»Ų“š£ŗ
A”¢B”¢C”¢XŹĒ֊ѧ»Æѧ³£¼ūĪļÖŹ£¬¾łÓɶĢÖÜĘŚŌŖĖŲ×é³É£¬×Ŗ»Æ¹ŲĻµČēĶ¼ĖłŹ¾£®ĒėÕė¶ŌŅŌĻĀČżÖÖ²»Ķ¬Ēéæö»Ų“š£ŗ

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 A”¢B”¢C”¢XŹĒ֊ѧ»Æѧ֊³£¼ūµÄĪļÖŹ£¬ĖüĆĒÖ®¼äµÄ×Ŗ»Æ¹ŲĻµČēĶ¼ĖłŹ¾£Ø²æ·Ö²śĪļŅŃĀŌČ„£©£®Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
A”¢B”¢C”¢XŹĒ֊ѧ»Æѧ֊³£¼ūµÄĪļÖŹ£¬ĖüĆĒÖ®¼äµÄ×Ŗ»Æ¹ŲĻµČēĶ¼ĖłŹ¾£Ø²æ·Ö²śĪļŅŃĀŌČ„£©£®Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 A”¢B”¢C”¢XŹĒ֊ѧ»Æѧ֊³£¼ūµÄĪļÖŹ£¬ĖüĆĒÖ®¼äµÄ×Ŗ»Æ¹ŲĻµČēĻĀĶ¼ĖłŹ¾£Ø²æ·Ö²śĪļŅŃĀŌČ„£©£®Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
A”¢B”¢C”¢XŹĒ֊ѧ»Æѧ֊³£¼ūµÄĪļÖŹ£¬ĖüĆĒÖ®¼äµÄ×Ŗ»Æ¹ŲĻµČēĻĀĶ¼ĖłŹ¾£Ø²æ·Ö²śĪļŅŃĀŌČ„£©£®Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com