��2011?�Ĵ�ģ�⣩ʯ����������к������⣬Ϊ����Ч��ֹ��Ⱦ��ʵ�ַ�����ۺ����ã���ҵ�ϲ������й��չ��̽�����ת��Ϊ����������Ȳ�Ʒ��
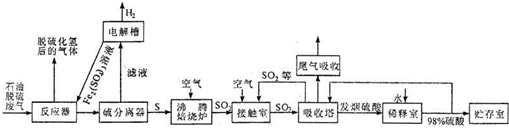
��ش��������⣺
��1����Ӧ����װ����������������Һ������Һ�����ⷴӦ�����ӷ���ʽ��
2Fe3++H2S=2Fe2++2H++S��
2Fe3++H2S=2Fe2++2H++S��
��
��2����Һ���õ���������������Һ���ٽ��䷵�ص���Ӧ���е�Ŀ����
ʹ��������������Һѭ��ʹ��
ʹ��������������Һѭ��ʹ��
��
��3����ʵ�������У���������ת��Ϊ������������������ǣ�V
2O
5����������ѹ��400�桫500�森��ͼ�Dz�ͬѹǿ�¸÷�Ӧ��ϵ���¶�����������ƽ��ת���ʱ仯���ߣ�
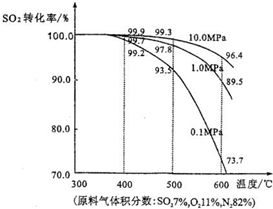
���ǹ�ҵ����Ч�沢���ͼʾ������ѡ��400�桫500���ԭ����
�ڴ��¶��£���Ӧ���ʺ�ƽ��ת���ʶ��ϸ�
�ڴ��¶��£���Ӧ���ʺ�ƽ��ת���ʶ��ϸ�
��
ѡ��ѹ��ԭ����
��ѹ�£�ƽ��ת���ʽϸߣ��Ҳ������ѹ����豸��ۺͺ�������
��ѹ�£�ƽ��ת���ʽϸߣ��Ҳ������ѹ����豸��ۺͺ�������
��
��4����ҵ�ϳ��ù�����ˮ����β���еĶ������÷�Ӧ�Ļ�ѧ����ʽ��
SO2+2NH3?H2O=��NH4��2SO3+H2O
SO2+2NH3?H2O=��NH4��2SO3+H2O
��
��5�����������У��Ӵ������ɵ�����������98%���������գ��Ƶ�һ�ַ������ᣨ9H
2SO
4?SO
3����ij���ᳵ��10Сʱ���ĵ���Ϊat����ƽ��ÿСʱ�������������ĸ÷�������Ϊ
t�������������������ʧ���Բ��ƣ���





 ������ϰ�ο����뵥Ԫ���ϵ�д�
������ϰ�ο����뵥Ԫ���ϵ�д�
 ��2011?�Ĵ�ģ�⣩X��Y��Z�Ƕ�����Ԫ�أ������ڱ��е�λ�ù�ϵ��ͼ��ʾ������˵������ȷ���ǣ�������
��2011?�Ĵ�ģ�⣩X��Y��Z�Ƕ�����Ԫ�أ������ڱ��е�λ�ù�ϵ��ͼ��ʾ������˵������ȷ���ǣ�������