
Θ®ΟΩΩ’2Ζ÷Θ§Ι≤6Ζ÷Θ©»»Μ·―ßΖΫ≥Χ Ϋ÷–ΒΡΠΛH ΒΦ …œ «»»ΝΠ―ß÷–ΒΡ“ΜΗωΈοάμΝΩΘ§Ϋ–Ήωλ ±δΘ§Τδ ΐ÷ΒΚΆΖϊΚ≈”κΖ¥”ΠΈοΚΆ…ζ≥…ΈοΒΡΉήΡήΝΩ”–ΙΊΘ§“≤”κΖ¥”ΠΈοΚΆ…ζ≥…ΈοΒΡΦϋΡή”–ΙΊΓΘ
(1)»γœ¬ΆΦΔώΥυ Ψ±μ ΨΒΡ «NO2ΚΆCOΖ¥”Π…ζ≥…CO2ΚΆNOΙΐ≥Χ÷–ΡήΝΩ±δΜ· Ψ“βΆΦΘ§«κ–¥≥ωNO2ΚΆCOΖ¥”ΠΒΡ»»Μ·―ßΖΫ≥Χ ΫΘΚ________________________ΓΘ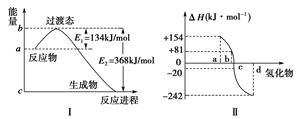
(2)ΆΦΔρ±μ Ψ―θΉε‘ΣΥΊ÷–ΒΡ―θΓΔΝρΓΔΈχΓΔμΎ‘Ύ…ζ≥…«βΜ·Έο ±ΒΡλ ±δ ΐΨίΘ§ΗυΨίλ ±δ ΐΨίΩ…»ΖΕ®aΓΔbΓΔcΓΔdΖ÷±π¥ζ±μΡΡ÷÷‘ΣΥΊΘ§ ‘–¥≥ωΈχΜ·«β‘Ύ»»ΝΠ―ß±ξΉΦΧ§œ¬Θ§ΖΔ…ζΖ÷ΫβΖ¥”ΠΒΡ»»Μ·―ßΖΫ≥Χ ΫΘΚ_________________________________________________ΓΘ
(3)“―÷ΣΘΚ
ΔΌFe2O3(s)ΘΪ3CO(g)===2Fe(s)ΘΪ3CO2(g)ΘΜ
ΠΛHΘΫΘ≠25 kJΓΛmolΘ≠1Θ§
ΔΎ3Fe2O3(s)ΘΪCO(g)===2Fe3O4(s)ΘΪCO2(g)ΘΜ
ΠΛHΘΫΘ≠47 kJΓΛmolΘ≠1Θ§
ΔέFe3O4(s)ΘΪCO(g)===3FeO(s)ΘΪCO2(g)ΘΜ
ΠΛHΘΫ19 kJΓΛmolΘ≠1
«κ–¥≥ωCOΜΙ‘≠FeOΒΡ»»Μ·―ßΖΫ≥Χ ΫΘΚ
________________________________________________________________________ΓΘ
(1)NO2(g)ΘΪCO(g)===NO(g)ΘΪCO2(g)ΘΜΠΛHΘΫΘ≠234 kJΓΛmolΘ≠1
(2)H2Se(g)===Se(g)ΘΪH2(g)ΘΜΠΛHΘΫ+81 kJ/mol
(3)FeO(s)ΘΪCO(g)===Fe(s)ΘΪCO2(g)ΘΜΠΛHΘΫΘ≠11 kJΓΛmolΘ≠1
ΫβΈω ‘ΧβΖ÷ΈωΘΚΘ®1Θ©’ΐ»Ζ ι–¥»»Μ·―ßΖΫ≥Χ ΫΘ§?“Σ÷ΗΟςΖ¥”Π ±ΒΡΈ¬Ε»ΚΆ―Ι«ΩΘΜ?ΖΫ≥Χ Ϋ÷–Υυ”–ΒΡΖ¥”ΠΈοΚΆ≤ζΈοΕΦ“Σ”Οά®Κ≈ΉΔΟςΥϋΟ«‘ΎΖ¥”Π ±ΒΡΉ¥Χ§ΘΜ?–¥≥ωΖ¥”ΠΒΡλ ±δΘ§ΉΔ“βλ ±δΒΡ’ΐΗΚΚ≈ΓΘΘ®2Θ©ΗυΨί‘ΣΥΊ÷ήΤΎ¬…Ω…÷Σ―θΉε‘ΣΥΊΒΡ―θΓΔΝρΓΔΈχΓΔμΎ‘Ύ…ζ≥…«βΜ·Έο ±”…“Ή±δΡ―Θ§Ζ¥”Π”…Ζ≈»»Ζ¥”Π±δΈΣΈϋ»»Ζ¥”ΠΘ§Υυ“‘a--μΎΘ§b--ΈχΘΜc--ΝρΘΜd--―θΘΜ”…ΆΦΩ…Ω¥≥ωΈχΖΔ…ζΖ¥”ΠΒΡλ ±δΈΣ+80KJ/molΓΘΘ®3Θ©ΫΪ?ΓΝ3ΓΣΘ®?+?ΓΝ2Θ©‘Ό≥ΐ“‘6Φ¥Ω…ΒΟΒΫCOΜΙ‘≠FeOΒΡ»»Μ·―ßΖΫ≥Χ ΫΚΆΖ¥”Πλ ±δ
ΩΦΒψΘΚ»»Μ·―ßΖΫ≥Χ ΫΒΡ ι–¥ΓΔΜ·―ßΖ¥”Πλ ±δΒΡ≈–ΕœΓΔΜ·―ßΖ¥”Π»»ΒΡΦΤΥψ
ΒψΤάΘΚ±ΨΧβ τ”Ύ±»ΫœΜυ¥ΓΒΡΧνΩ’ΧβΘ§»»Μ·―ß÷Σ Ε‘ΎΗΏΩΦ÷–’Φ”–±»Ϋœ÷Ί“ΣΒΡΈΜ÷Ο



| ΡξΦΕ | ΗΏ÷–ΩΈ≥Χ | ΡξΦΕ | ≥θ÷–ΩΈ≥Χ |
| ΗΏ“Μ | ΗΏ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ“Μ | ≥θ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏΕΰ | ΗΏΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θΕΰ | ≥θΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏ»ΐ | ΗΏ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ»ΐ | ≥θ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
“Μ÷÷»ή“ΚάοΩ…ΡήΚ§”–OHΘ≠ΓΔClΘ≠ΓΔCO![]() ΓΔSO
ΓΔSO![]() ΥΡ÷÷άκΉ”÷–ΒΡ“Μ÷÷ΜρΦΗ÷÷ΓΘ»Γ…ΌΝΩ’β÷÷»ή“ΚΖ÷±π Δ”Ύ»ΐ÷ß ‘Ιή÷–Θ§Ϋχ––»γœ¬ Β―ιΘΚ
ΥΡ÷÷άκΉ”÷–ΒΡ“Μ÷÷ΜρΦΗ÷÷ΓΘ»Γ…ΌΝΩ’β÷÷»ή“ΚΖ÷±π Δ”Ύ»ΐ÷ß ‘Ιή÷–Θ§Ϋχ––»γœ¬ Β―ιΘΚ
Θ®1Θ©œρΒΎ“Μ÷ß ‘Ιή÷–ΒΈ»κΖ”ΧΣ ‘“ΚΘ§»ή“Κ±δΚλΓΘ
Θ®2Θ©œρΒΎΕΰ÷ß ‘Ιή÷–Φ”»κBaΘ®NO3Θ©2»ή“ΚΘ§”–ΑΉ…Ϊ≥ΝΒμ…ζ≥…ΓΘ
Θ®3Θ©œρΒΎ»ΐ÷ß ‘Ιή÷–÷πΒΈΦ”»κœΓHNO3»ή“ΚΘ§”–Έό…ΪΤχΧε…ζ≥…Θ§ΗΟΤχΧεΡή Ι≥Έ«ε ·Μ“Υ°±δΜκΉ«ΘΜΦΧ–χΦ”»κœΓHNO3 Ι»ή“Κ≥ Υα–‘ ±Θ§‘ΌΦ”»κBaΘ®NO3Θ©2»ή“Κ≤Μ≤ζ…ζ≥ΝΒμΓΘ
‘≈–ΕœΘΚΗΟ»ή“Κ÷–ΩœΕ®Κ§”–___________άκΉ”Θ§ΩœΕ®ΟΜ”–___________άκΉ”Θ§≤ΜΡήΩœΕ®”–ΟΜ”–ΒΡ «_________άκΉ”ΓΘΘ®ΟΩΩ’2Ζ÷Θ§Ι≤6Ζ÷Θ©
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2012-2013―ßΡξΫ≠Ές ΓΑ≤ΗΘ÷–―ßΗΏ“ΜΒΎ“Μ¥Έ‘¬ΩΦΜ·―ß ‘ΨμΘ®¥χΫβΈωΘ© Χβ–ΆΘΚΧνΩ’Χβ
Θ®ΟΩΩ’2Ζ÷Θ§Ι≤6Ζ÷Θ©Θ°12.4g Na2R Κ§”–Na+0.4molΘ§‘ρNa2RΒΡΡΠΕϊ÷ ΝΩΈΣ Θ§RΒΡœύΕ‘‘≠Ή”÷ ΝΩΈΣ Θ§Κ§R1.6g ΒΡNa2RΒΡΈο÷ ΒΡΝΩΈΣ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2014Ϋλ‘ΤΡœ ΓΗΏΕΰ…œ―ßΤΎΤΎΡ©ΩΦ ‘Μ·―ß ‘ΨμΘ®ΫβΈωΑφΘ© Χβ–ΆΘΚΧνΩ’Χβ
Θ®ΟΩΩ’2Ζ÷Θ§Ι≤6Ζ÷Θ©»»Μ·―ßΖΫ≥Χ Ϋ÷–ΒΡΠΛH ΒΦ …œ «»»ΝΠ―ß÷–ΒΡ“ΜΗωΈοάμΝΩΘ§Ϋ–Ήωλ ±δΘ§Τδ ΐ÷ΒΚΆΖϊΚ≈”κΖ¥”ΠΈοΚΆ…ζ≥…ΈοΒΡΉήΡήΝΩ”–ΙΊΘ§“≤”κΖ¥”ΠΈοΚΆ…ζ≥…ΈοΒΡΦϋΡή”–ΙΊΓΘ
(1)»γœ¬ΆΦΔώΥυ Ψ±μ ΨΒΡ «NO2ΚΆCOΖ¥”Π…ζ≥…CO2ΚΆNOΙΐ≥Χ÷–ΡήΝΩ±δΜ· Ψ“βΆΦΘ§«κ–¥≥ωNO2ΚΆCOΖ¥”ΠΒΡ»»Μ·―ßΖΫ≥Χ ΫΘΚ________________________ΓΘ
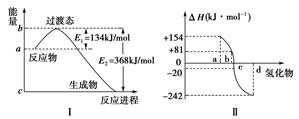
(2)ΆΦΔρ±μ Ψ―θΉε‘ΣΥΊ÷–ΒΡ―θΓΔΝρΓΔΈχΓΔμΎ‘Ύ…ζ≥…«βΜ·Έο ±ΒΡλ ±δ ΐΨίΘ§ΗυΨίλ ±δ ΐΨίΩ…»ΖΕ®aΓΔbΓΔcΓΔdΖ÷±π¥ζ±μΡΡ÷÷‘ΣΥΊΘ§ ‘–¥≥ωΈχΜ·«β‘Ύ»»ΝΠ―ß±ξΉΦΧ§œ¬Θ§ΖΔ…ζΖ÷ΫβΖ¥”ΠΒΡ»»Μ·―ßΖΫ≥Χ ΫΘΚ_________________________________________________ΓΘ
(3)“―÷ΣΘΚ
ΔΌFe2O3(s)ΘΪ3CO(g)===2Fe(s)ΘΪ3CO2(g)ΘΜ
ΠΛHΘΫΘ≠25 kJΓΛmolΘ≠1Θ§
ΔΎ3Fe2O3(s)ΘΪCO(g)===2Fe3O4(s)ΘΪCO2(g)ΘΜ
ΠΛHΘΫΘ≠47 kJΓΛmolΘ≠1Θ§
ΔέFe3O4(s)ΘΪCO(g)===3FeO(s)ΘΪCO2(g)ΘΜ
ΠΛHΘΫ19 kJΓΛmolΘ≠1
«κ–¥≥ωCOΜΙ‘≠FeOΒΡ»»Μ·―ßΖΫ≥Χ ΫΘΚ
________________________________________________________________________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2015ΫλΫ≠Ές ΓΗΏ“ΜΒΎ“Μ¥Έ‘¬ΩΦΜ·―ß ‘ΨμΘ®ΫβΈωΑφΘ© Χβ–ΆΘΚΧνΩ’Χβ
Θ®ΟΩΩ’2Ζ÷Θ§Ι≤6Ζ÷Θ©Θ°12.4g Na2R Κ§”–Na+0.4molΘ§‘ρNa2RΒΡΡΠΕϊ÷ ΝΩΈΣ Θ§RΒΡœύΕ‘‘≠Ή”÷ ΝΩΈΣ Θ§Κ§R1.6g ΒΡNa2RΒΡΈο÷ ΒΡΝΩΈΣ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΑΌΕ»÷¬–≈ - ΝΖœΑ≤αΝ–±μ - ‘ΧβΝ–±μ
Κΰ±± ΓΜΞΝΣΆχΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΤΫΧ® | Άχ…œ”–ΚΠ–≈œΔΨΌ±®Ή®«χ | Βγ–≈’©Τ≠ΨΌ±®Ή®«χ | …φάζ Ζ–ιΈό÷ς“ε”–ΚΠ–≈œΔΨΌ±®Ή®«χ | …φΤσ«÷»®ΨΌ±®Ή®«χ
ΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΒγΜΑΘΚ027-86699610 ΨΌ±®” œδΘΚ58377363@163.com