

���� þ���ܼӦ����NaOH��Һ�Ƚ�þ�����벢�õ�ƫ��������Һ������Һ�����ữ�õ������������������Ǻ��ܽ���ϡ�����У������������Һ���������ᾧ���ȵ�Ŀ����
��1��������Ϊǿ����ʣ�
��2����þ���Ͻ��м�����������������Һ��Al������������Һ���ɿ����Ե�ƫ�����ƣ�þ����Ӧ����Һ�к���NaAlO2����������������
��3������������IJ���������Ũ������ȴ�ᾧ�����½ᾧ��������ϴ�ӡ����
��4��þ�Ͷ�����̼�ڵ�ȼ�����·�Ӧ��������þ��̼��
��5��ij��ȤС��Ϊ�ⶨþ���Ͻ��и���ɵ���������������ͼ��֪���ܹ����������������ݽ���������֮��Ĺ�ϵʽ����֪�����ͼ1ʾװ����Ҫ�ⶨ�������������������þ���Ͻ��������
��� �⣺þ���ܼӦ����NaOH��Һ�Ƚ�þ�����벢�õ�ƫ��������Һ������Һ�����ữ�õ������������������Ǻ��ܽ���ϡ�����У������������Һ���������ᾧ���ȵ�Ŀ����
��1��������Ϊǿ����ʣ���ˮ�еĵ��뷽��ʽΪAl2��SO4��3=2Al3++3SO42-���ʴ�Ϊ��Al2��SO4��3=2Al3++3SO42-��
��2����þ���Ͻ��м�����������������Һ��Al������������Һ���ɿ����Ե�ƫ�����ƣ�þ����Ӧ��������Ӧ�����ӷ���ʽΪ2Al+2OH-+2H2O=2AlO2-+3H2����
��Һ�к���NaAlO2��ͨ������CO2�Ļ�ѧ����ʽΪNaAlO2+CO2+2H2O=Al��OH��3+NaHCO3��
�ʴ�Ϊ��2Al+2OH-+2H2O=2AlO2-+3H2����NaAlO2+CO2+2H2O=Al��OH��3+NaHCO3��
��3������������IJ���������Ũ������ȴ�ᾧ�����½ᾧ��������ϴ�ӡ����
�ʴ�Ϊ����ȴ�ᾧ�����½ᾧ����
��4��þ�Ͷ�����̼�ڵ�ȼ�����·�Ӧ��������þ��̼����Ӧ�Ļ�ѧ����ʽΪ2Mg+CO2$\frac{\underline{\;��ȼ\;}}{\;}$2 MgO+C��
�ʴ�Ϊ��2Mg+CO2$\frac{\underline{\;��ȼ\;}}{\;}$2 MgO+C��
��5��ij��ȤС��Ϊ�ⶨþ���Ͻ��и���ɵ���������������ͼ��֪���ܹ����������������ݽ���������֮��Ĺ�ϵʽ����֪�����ͼ1ʾװ����Ҫ�ⶨ�������������������þ���Ͻ��������
�ʴ�Ϊ�������������þ���Ͻ��������
���� ���⿼���Ʊ�ʵ�鷽����ƣ�Ϊ�߿���Ƶ�㣬���������漰�������ʡ�ʵ�������������ȷ���ʵ������Լ��������������ǽⱾ��ؼ�����������Ԫ�ػ�����֪ʶ���������֪ʶ������⣬��Ŀ�Ѷ��еȣ�


 һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�
һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 7.2gC5H12���й��ۼ���Ϊ1.6NA | |
| B�� | 14g��ϩ�ͱ�ϩ�Ļ�����к���ԭ�ӵ���ĿΪNA | |
| C�� | ��״���£�224mL������ȼ�պ����ɵ�CO2�ķ�����Ϊ0.06NA | |
| D�� | 1.7g�ǻ���-OH�����еĵ�����ΪNA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
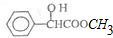
| A�� | ����ʽΪC9H10O3 | |
| B�� | ���Է���ȡ����Ӧ���ӳɷ�Ӧ��������Ӧ�ͻ�ԭ��Ӧ | |
| C�� | 1mol������������4mol H2�����ӳɷ�Ӧ | |
| D�� | �䱽���ϵĶ��ȴ��ﹲ�����ֽṹ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
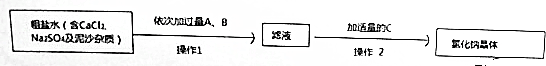
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
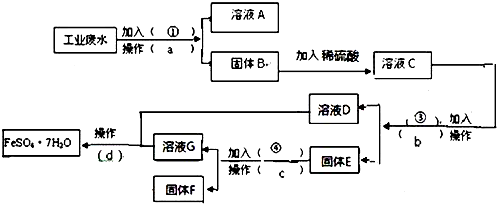
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �й��Ŵ�����������Һ���������ͭ�������ͭ�� | |
| B�� | ��ͥ�õġ�84������Һ�������ܻ��ʹ�ã����ᷢ���ж��¹� | |
| C�� | ����պ��Ũ��ˮ�IJ������������������Ĺܵ��Ƿ�©�� | |
| D�� | �轺������ʳƷ����� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com