
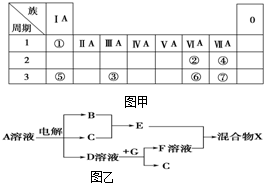
 ��ͼ��ΪԪ�����ڱ���һ���֣�����Ԫ�آ١����ڱ��е�λ�ã����û�ѧ����ش��������⣺
��ͼ��ΪԪ�����ڱ���һ���֣�����Ԫ�آ١����ڱ��е�λ�ã����û�ѧ����ش��������⣺
| ||
| ||


 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
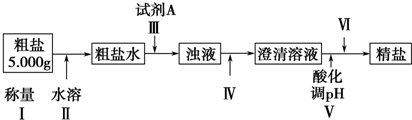
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
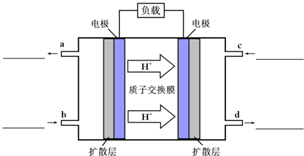 �״�-����ȼ�ϵ�أ�DMFC����һ�ָ�Ч�ܡ�����Ⱦ�綯�����ij��ص�أ���ȼ�ϵ�صĵ�ط�ӦʽΪ��CH3OH��l��+
�״�-����ȼ�ϵ�أ�DMFC����һ�ָ�Ч�ܡ�����Ⱦ�綯�����ij��ص�أ���ȼ�ϵ�صĵ�ط�ӦʽΪ��CH3OH��l��+| 3 |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
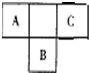 ������Ԫ�ص�A��B��C��Ԫ�����ڱ��е�λ������ͼ��ʾ����֪A��C ����Ԫ�ص�ԭ�Ӻ��������֮�͵���B����������Bԭ�Ӻ�������������������ȣ��ݴ���գ�
������Ԫ�ص�A��B��C��Ԫ�����ڱ��е�λ������ͼ��ʾ����֪A��C ����Ԫ�ص�ԭ�Ӻ��������֮�͵���B����������Bԭ�Ӻ�������������������ȣ��ݴ���գ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��ԭ�������n��Fe����n��Fe2O3��=2��1 |
| B������Һ�е�����ɫ��KSCN��Һ����Ѫ��ɫ |
| C���������ԭ���������� |
| D����ʱ��Һ��Fe2+��Fe3+�����ʵ���֮��Ϊ3��1 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com