
������Ϊһ������Դ�ڻ�ѧ����Ӧ�ù㷺����ش��������⣺
(1)��¯ұ�������У������ڴ���Ӧ���в���ˮú��(CO��H2)��ԭ���������йط�ӦΪ��CH4(g)��CO2(g)=2CO(g)��2H2(g)����H����260 kJ��mol��1
��֪��2CO(g)��O2(g)=2CO2(g) ��H����566 kJ��mol��1
��CH4��O2��Ӧ����CO��H2���Ȼ�ѧ����ʽΪ_______________________________
(2)����ͼ��ʾ��װ�â�Ϊ����ȼ�ϵ��(�������ҺΪKOH��Һ)��ͨ��װ�â�ʵ�������϶�ͭ��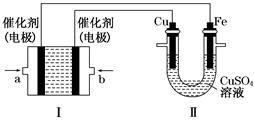
��a��Ӧͨ��________(�CH4����O2��)��b���缫�Ϸ����ĵ缫��Ӧʽ��______________________________
�ڵ�ƽ�����װ�â�����Һ��pH________(��д�������С�����䡱����ͬ)��װ�â���Cu2�������ʵ���Ũ��________��
�۵�ƽ�����װ�â���Һ�е������ӳ���OH���������________(����ˮ��)��
���ڴ˹���������ȫ��Ӧ��װ�â������������仯12.8 g����װ�â������������ļ���________L(��״����)��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��14�֣������������ҹ��ж��������������ص��������������ȫ����ڵ������쳣�����������ٴγ�Ϊ���㡣�ǽ���������ĺ������ƺ��������Ż��������滷������Ч;��֮һ�������û�ѧ��Ӧԭ��֪ʶ���ش��������⣺
��Ŀǰ����������������Ⱦ�ж��ַ�����
��1����CH4����ԭ��������������������������Ⱦ����֪��
��CH4��g��+4NO2��g����4NO��g��+CO2��g��+2H2O��g�� ��H����57kJ?mol-1
��4CH4��g��+4NO��g����2N2��g��+CO2��g��+2H2O��g������H����1160kJ?mol-1
��H2O��g����H2O��l�� ��H����44.0kJ?mol-1
д��CH4��g����NO2��g����Ӧ����N2��g����CO2��g����H2O��l�����Ȼ�ѧ����ʽ________________��
��2���û���̿��ԭ��������������йط�ӦΪ��C(s)+ 2NO��g�� N2��g��+CO2��g��ij�о�С��������ܱ������м���һ�����Ļ���̿��NO�����£�T�棩�����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
N2��g��+CO2��g��ij�о�С��������ܱ������м���һ�����Ļ���̿��NO�����£�T�棩�����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
| Ũ�ȣ�mol/L�� ʱ�䣨min�� | NO | N2 | CO2 |
| 0 | 0.100 | 0 | 0 |
| 10 | 0.058 | 0.021 | 0.021 |
| 20 | 0.040 | 0.030 | 0.030 |
| 30 | 0.040 | 0.030 | 0.030 |
| 40 | 0.032 | 0.034 | 0.017 |
| 50 | 0.032 | 0.034 | 0.017 |
| n(SO32��)��n(HSO3��) | 91��9 | 1��1 | 1��91 |
| pH | 8.2 | 7.2 | 6.2 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�״���һ����;�㷺�Ļ���ԭ�ϡ�
��1����ҵ�ϳ����������ַ�Ӧ�Ʊ��״���
��CO(g) + 2H2(g)  CH3OH(g) ��H1= -90��1KJ/mol
CH3OH(g) ��H1= -90��1KJ/mol
��CO2(g)�� 3H2(g)  CH3OH(g) + H2O(l) ��H2
CH3OH(g) + H2O(l) ��H2
��֪��CO(g)+ H2O (g) �� CO2 (g) + H2 (g) ��H3=-41��1 KJ/mol ��
H2O (l) ��H2O (g) ��H4=+44��0KJ/mol ��
��H2��
��2��ʵ����ģ����CO��H2��Ӧ���Ƽ״�����250���£���һ������CO��H2Ͷ��10L���ܱ������У������ʵ����ʵ���Ũ��(mol?L-1)�仯���±���ʾ����ǰ6minû�иı�������
| | 2min | 4min | 6min | 8min | �� |
| CO | 0��07 | 0��06 | 0��06 | 0��05 | �� |
| H2 | x | 0��12 | 0��12 | 0��2 | �� |
| CH3OH | 0��03 | 0��04 | 0��04 | 0��05 | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����һ�������£���ѧ�����ô��̵����з����CO2��̫���ܵ�ص��ˮ������H2�ϳɼ״������������ͼ��ʾ���Իش��������⣺
��1���úϳ�·�߶��ڻ��������ļ�ֵ���� ��
��2��15��20%���Ҵ�����HOCH2CH2NH2��ˮ��Һ���������ԣ������ϳ���·������CO2���ռ��������ӷ���ʽ��ʾ�Ҵ���ˮ��Һ�������Ե�ԭ�� ��
��3��CH3OH��H2��ȼ���ȷֱ�Ϊ����H����725.5 kJ/mol����H����285.8 kJ/mol��д����ҵ����CO2��H2�ϳ�CH3OH���Ȼ�ѧ����ʽ�� ��
��ȼú�����е�CO2ת��Ϊ���ѵķ�Ӧԭ��Ϊ��
2CO2(g) + 6H2(g)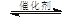 CH3OCH3(g) + 3H2O(g)
CH3OCH3(g) + 3H2O(g)
��֪һ��ѹǿ�£��÷�Ӧ�ڲ�ͬ�¶ȡ���ͬͶ�ϱ�ʱ��CO2��ת���ʼ��±���
| Ͷ�ϱ�[n(H2) / n(CO2)] | 500 K | 600 K | 700 K | 800 K |
| 1.5 | 45% | 33% | 20% | 12% |
| 2.0 | 60% | 43% | 28% | 15% |
| 3.0 | 83% | 62% | 37% | 22% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����β���е�NOx�Ǵ�����Ⱦ��֮һ����ѧ�����ڳ����ø���ѧ�ķ�����NOxת���������ʣ��Ӷ���������β����Ⱦ��
��1��ѹ����Ȼ����CNG���������ŵ�֮һ�����ô������ܹ���NOxת�������CO2��N2��
��CH4(g)��4NO2(g)  4NO(g)��CO2(g)��2H2O(g) ��H1��0
4NO(g)��CO2(g)��2H2O(g) ��H1��0
��CH4(g)��4NO(g) 2N2(g)��CO2(g)��2H2O(g) ��H2��0
2N2(g)��CO2(g)��2H2O(g) ��H2��0
��CH4(g) ��2NO2(g) N2(g) ��CO2(g) ��2H2O(g) ��H3�� �����á�H1�͡�H2��ʾ��
N2(g) ��CO2(g) ��2H2O(g) ��H3�� �����á�H1�͡�H2��ʾ��
��2���ں�ѹ�£���CH4(g)��NO2(g)�����ܱ������з�����ѧ��Ӧ�ۣ��ڲ�ͬ�¶ȡ���ͬͶ�ϱ�ʱ��NO2��ƽ��ת���ʼ��±���
| Ͷ�ϱ�[n(NO2) / n(CH4)] | 400 K | 500 K | 600 K |
| 1 | 60% | 43% | 28% |
| 2 | 45% | 33% | 20% |


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
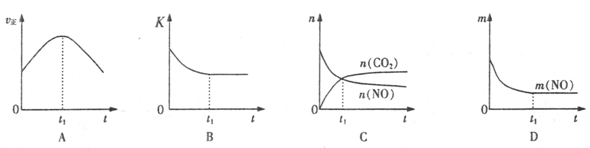
2013�����ȫ�����ض�����ж�������ʮ������������ɡ������족����Ҫ��Դ֮һ������β����ȼúβ���ŷų����Ĺ���С������
����β����������Ҫԭ��Ϊ��2NO(g)+2CO(g)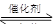 2CO2+N2�����ܱ������з����÷�Ӧʱ��c(CO2)���¶�(T)�������ı����(S)��ʱ��(t)�ı仯��������ͼ��ʾ���ݴ��жϣ�
2CO2+N2�����ܱ������з����÷�Ӧʱ��c(CO2)���¶�(T)�������ı����(S)��ʱ��(t)�ı仯��������ͼ��ʾ���ݴ��жϣ� 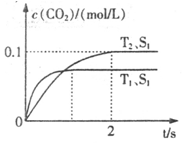
��1���÷�ӦΪ ��Ӧ(����ȡ������ȡ�������T2�¶��£�0~2s�ڵ�ƽ����Ӧ���ʣ�v(N2)= ����2�����������������һ��ʱ�����������������ѧ��Ӧ���ʡ��������ı����S1>S2���ڴ���ϻ��� c(CO2)��T1��S2�����´ﵽƽ������еı仯���ߡ�
��3��ij���л�������t1���£�����㶨���ܱ������У������崫��������˲�ͬʱ���NO��CO��Ũ�ȣ��������ݼ��±���CO2��N2����ʼŨ��Ϊ0����
| ʱ��/s | 0 | 1 | 2 | 3 | 4 | 5 |
| c(NO)/xl0-4mol L-1 | 10��0 | 4��50 | 2��50 | 1��50 | 1��00 | 1��00 |
| c(CO)/xl0-3mol L-1 | 3��60 | 3��05 | 2��85 | 2��75 | 2��70 | 2��70 |
 N2O4 (g) ��H=-56��9kJ ? mol-1
N2O4 (g) ��H=-56��9kJ ? mol-1�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪H+(aq)+OH-(aq) H2O(l)����H="-57.3" kJ��mol-1,�ش��������⡣
H2O(l)����H="-57.3" kJ��mol-1,�ش��������⡣
(1)�ú�20 g NaOH��ϡ��Һ������ϡ���ᷴӦ�ų���������kJ��������
(2)�ú�2 mol H2SO4��ϡ��Һ������ϡNaOH��Ӧ,�˷�Ӧ���к���Ϊ��������������
(3)�����(1)��Ӧ�е�ϡ���ỻ��ϡ����,��Ӧ�ų���������������������(����ڡ���С�ڡ����ڡ�)ԭ��(1)�ų���������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪���ƻ�1 mol N��N����H��H����N��H���ֱ���Ҫ���յ�����Ϊ946 kJ��436 kJ��391 kJ������1 mol N2(g)��3 mol H2(g)��ȫת��ΪNH3(g)�������仯����ֵΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�Ķ�������Ϣ���ش����⣺
��1����֪NO2��N2O4�Ľṹʽ�ֱ�Ϊ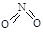 ��
��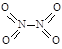 �� NO2�е������ļ���Ϊ466 kJ��mol��1��N2O4��N��N������Ϊ167 kJ��mol��1���������ļ���Ϊ438.5 kJ��mol��1��д��N2O4ת��ΪNO2���Ȼ�ѧ����ʽ ��
�� NO2�е������ļ���Ϊ466 kJ��mol��1��N2O4��N��N������Ϊ167 kJ��mol��1���������ļ���Ϊ438.5 kJ��mol��1��д��N2O4ת��ΪNO2���Ȼ�ѧ����ʽ ��
��2��ij�ָ��ܳ����ʹ������H2��Ĵ���Ͻ���MH��ʾ������ظ������ϣ�NiO(OH)���������ϣ�KOH��ҺΪ�������Һ�������ĵ缫��ӦΪ��MH��OH����e��= M��H2O����س�ŵ�ʱ���ܷ�ӦΪ��Ni(OH)2��M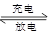 NiO(OH)��MH
NiO(OH)��MH
�� ��طŵ�ʱ�������ĵ缫��ӦʽΪ ��
�� ������ʱNi(OH)2ȫ��ת��ΪNiO(OH)����������罫��һ���缫����O2��ͬʱ��ɢ����һ���缫�����缫��Ӧ�����ģ���ʱ�����ĵ缫��ӦʽΪ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com