
ijʯ��Һ�����ɱ���Ͷ�����ɣ������������ֱ�Ϊ80����20��������ȼ�յ��Ȼ�ѧ����ʽ�ֱ�Ϊ��
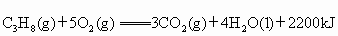
(��ʽ��ʾ1mol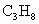 ������ȫȼ������3mol
������ȫȼ������3mol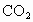 �����4molҺ̬ˮʱ���ų�2200kJ������)��
�����4molҺ̬ˮʱ���ų�2200kJ������)��
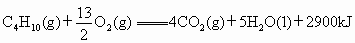
(��ʽ��ʾ1mol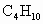 ������ȫȼ������4mol
������ȫȼ������4mol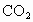 �����5molҺ̬ˮʱ���ų�2900kJ������)����һ����Ϊ0.80kg���ݻ�Ϊ4.0L����������һ��20���ˮ�տ�������Һ��ʯ����0.056kg���Լ����ȼ�ϵ������ʣ�[��֪ˮ�ı���Ϊ4.2kJ/(kg����)�����ı���Ϊ0.88kJ/(kg����)]
�����5molҺ̬ˮʱ���ų�2900kJ������)����һ����Ϊ0.80kg���ݻ�Ϊ4.0L����������һ��20���ˮ�տ�������Һ��ʯ����0.056kg���Լ����ȼ�ϵ������ʣ�[��֪ˮ�ı���Ϊ4.2kJ/(kg����)�����ı���Ϊ0.88kJ/(kg����)]

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
C4H10(g)+![]() O2(g)====4CO2(g)+5H2O(l)����H=-2 900 kJ��mol-1��
O2(g)====4CO2(g)+5H2O(l)����H=-2 900 kJ��mol-1��
��һ����Ϊ0.80 kg���ݻ�Ϊ4.0 L����������һ��20 ���ˮ�տ�������Һ��ʯ����0.056 kg,�Լ����ȼ�ϵ������ʡ�
����֪ˮ�ı���Ϊ4.2 kJ��(kg����)-1�����ı���Ϊ0.88 kJ��(kg����)-1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijʯ��Һ�����ɱ���Ͷ�����ɣ������������ֱ�Ϊ80%��20%����֪��
C3H8(g)+5O2(g)![]() 3CO2(g)+4H2O(g) ��H=��2200kJ��mol-1
3CO2(g)+4H2O(g) ��H=��2200kJ��mol-1
2C4H10(g)+13O2(g)![]() 8CO2(g)+10H2O(g) ��H=��5800kJ��mol-1
8CO2(g)+10H2O(g) ��H=��5800kJ��mol-1
����һ����Ϊ0.80kg���ݻ�Ϊ4.0L����������һ��20���ˮ�տ�������0.056kgʯ��Һ�������Լ����ȼ�ϵ������ʡ���֪ˮ�ı�����Ϊ4.2kJ��kg-1����-1,���ı�����Ϊ0.88kJ��kg-1����-1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��15�֣�ijʯ��Һ�����ɱ���Ͷ�����ɣ������������ֱ�Ϊ80����20��������ȼ�յ��Ȼ�ѧ����ʽ�ֱ�Ϊ��
C3H8(g)+5O2(g)==3CO2(g)+4H2O(1) ��H��-2 200kJ��mol
C4H10(g)+O2(g)==4CO2(g)+5H2O(1) ��H��-2 900 kJ��mol
��һ����Ϊ0.80kg���ݻ�Ϊ4.0L����������һ��20���ˮ�տ�������Һ��ʯ����0.056kg���Լ����ȼ�ϵ������ʡ�[��֪ˮ�ı���Ϊ4.2kJ��(kg����)�����ı���Ϊ0.88kJ��(kg����)]
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�����ɹź��ױ���������ʯ��ҵһ�и�����ѧ�ڵ�һ��ģ�⿼�Ի�ѧ�Ծ� ���ͣ�������
��15�֣�ijʯ��Һ�����ɱ���Ͷ�����ɣ������������ֱ�Ϊ80����20��������ȼ�յ��Ȼ�ѧ����ʽ�ֱ�Ϊ��
C3H8(g)+5O2(g)==3CO2(g)+4H2O(1) ��H��-2 200kJ��mol
C4H10(g)+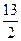 O2(g)==4CO2(g)+5H2O(1) ��H��-2 900 kJ��mol
O2(g)==4CO2(g)+5H2O(1) ��H��-2 900 kJ��mol
��һ����Ϊ0.80kg���ݻ�Ϊ4.0L����������һ��20���ˮ�տ�������Һ��ʯ����0.056kg���Լ����ȼ�ϵ������ʡ�[��֪ˮ�ı���Ϊ4.2kJ��(kg����)�����ı���Ϊ0.88kJ��(kg����)]
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com