
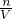 �ж϶�������ҺŨ��Ӱ�죮
�ж϶�������ҺŨ��Ӱ�죮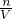 ������Һ��������������
������Һ�������������� 

 ��ɢ˼ά�¿���ϵ�д�
��ɢ˼ά�¿���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016���Ĵ�ʡ�����и�һ��ѧ��12���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
ʵ������NaCl������400 mL 1.0 mol��L NaCl��Һ�������жϲ��Ե��ǣ� ��
A����������ƽ��ȡNaCl����23.4 g
B��Ӧѡ��500 mL������ƿ�����ƴ���Һ
C����ת�Ʋ����в�������Һ��������ƿ���棬Ӧ������������Һ
D��������ˮ����̶���1-2 cmʱ���ý�ͷ�ιܶ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�긣��ʡ��������Ϫ���Ĺ���ѧ��һ���ϣ����л�ѧ�Ծ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com