
| A£®S(s)ŌŚO2(g)ÖŠČ¼Éյķ“Ó¦ŹĒ·ÅČČ·“Ó¦ |
| B£®S(g) + O2(g)= SO2(g)£»”÷H=bkJ”¤mol£1,Ōņa<b |
| C£®1mol SO2(g)Ėł¾ßÓŠµÄÄÜĮæµĶÓŚ1mol S(s)Óė1mol O2(g)Ėł¾ßÓŠµÄÄÜĮæÖ®ŗĶ |
| D£®16æĖ¹ĢĢåĮņŌŚæÕĘųÖŠ³ä·ÖČ¼ÉÕ£¬æɷųö148.6kJµÄČČĮæ |

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®Q1=Q2=Q3 | B£®Q2£¾Q1£¾Q3 | C£®Q2£¾Q3£¾Q1 | D£®Q2=Q3£¾Q1 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®1mol SĶźČ«Č¼ÉÕŹ±·Å³öµÄČČĮæĪŖ297£®23kJ |
| B£®S(g)+O2(g)=SO2(g)·Å³öµÄČČĮæ“óÓŚ297.23kJ |
| C£®S(g)+O2(g)=SO2(g)·Å³öµÄČČĮæŠ”ÓŚ297.23KJ |
| D£®ŠĪ³É1mol SO2µÄ»Æѧ¼üĖłŹĶ·ÅµÄ×ÜÄÜĮæ“óÓŚ¶ĻĮŃlmol S(s)ŗĶ1mol O2(g)µÄ»Æѧ¼üĖłĪüŹÕµÄ×ÜÄÜĮæ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
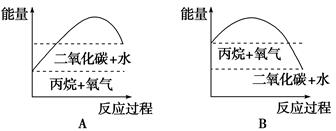
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗ¼ĘĖćĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
 2SO3(g) ”÷H=-196.64kJ/mol£¬µ±·Å³ö314.624kJČČĮæŹ±£¬SO2µÄ×Ŗ»ÆĀŹĪŖ£Ø £©
2SO3(g) ”÷H=-196.64kJ/mol£¬µ±·Å³ö314.624kJČČĮæŹ±£¬SO2µÄ×Ŗ»ÆĀŹĪŖ£Ø £©| A£®40% | B£®50% | C£®80% | D£®90% |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
 O2(g) == H2O(l) ”÷H3£½-285.8kJ”¤mol-1
O2(g) == H2O(l) ”÷H3£½-285.8kJ”¤mol-1| A£®-488.3 kJ”¤mol-1 | B£®-244.15 kJ”¤mol-1 | C£®+488.3 kJ”¤mol-1 | D£®+244.15 kJ”¤mol-1 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
| | ¼üÄÜ | | ¼üÄÜ | | ¼üÄÜ |
| H£H | 436 | Cl£Cl | 243 | H£Cl | 432 |
| S£½S | 255 | H£S | 339 | C£F | 427 |
| C£Cl | 330 | C£I | 218 | H£F | 565 |
| C£O | 347 | H£O | 464 | Si”ŖSi | 176 |
| Si”ŖO | 460 | O=O | 497 | | |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com