
£Ø2£©ŌŚ£Ø1£©ŹµŃéÖŠČōĘäĖū²Ł×÷¾łÕżČ·£¬µ«¶ØČŻŹ±ø©ŹÓæĢ¶ČĻߣ¬ŌņĖłµĆČÜŅŗÅضČ________0.2 mol”¤L-1”££ØĢī”°“óÓŚ”±”°
£Ø3£©ŌŚ£Ø1£©ŹµŃéÖŠČōNaOHČÜŅŗŌŚ×ŖŅĘÖĮČŻĮæĘæŹ±£¬Č÷ĀäĮĖÉŁŠķ£¬ŌņĖłµĆČÜŅŗÅضČ________0.2 mol”¤L-1”££ØĢī”°“óÓŚ”±”°µČÓŚ”±»ņ”°Š”ÓŚ”±£©
½āĪö£ŗ£Ø1£©øł¾ŻĢāÄæĢõ¼žæÉÖŖŠčŅŖNaOHµÄĪļÖŹµÄĮæĪŖ0
“š°ø£ŗ£Ø1£©4.0””Š”ÉÕ±””Š”ÉÕ±””ČÜŅŗĄäČ“””£Ø2£©“óÓŚ?
£Ø3£©Š”ÓŚ


 øßÖŠ±ŲĖ¢ĢāĻµĮŠ“š°ø
øßÖŠ±ŲĖ¢ĢāĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
8MnO![]() +5Cu2S+44H+
+5Cu2S+44H+![]() 10Cu2++5SO2+8Mn2++22H2O
10Cu2++5SO2+8Mn2++22H2O
6MnO![]() +5CuS+28H+
+5CuS+28H+![]() 5Cu2++5SO2+6Mn2++14H2O
5Cu2++5SO2+6Mn2++14H2O
·“Ó¦ŗóÖó·ŠČÜŅŗ£¬øĻ¾”SO2£¬Ź£ÓąµÄ KMnO4Ē”ŗĆÓė350 mL 0.1 mol”¤L-1(NH4)2Fe(SO4)2ČÜŅŗĶźČ«·“Ó¦”£?
(1)ÅäĘ½KMnO4Óė(NH4)2Fe(SO4)2·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ?
![]()
(2)KMnO4ČÜŅŗÓė»ģŗĻĪļ·“Ó¦ŗó£¬Ź£ÓąKMnO4µÄĪļÖŹµÄĮæĪŖ________mol”£
(3)ÓūÅäÖĘ500 mL 0.1 mol”¤L-1 Fe2+ČÜŅŗ£¬Šč³ĘČ”(NH4)2Fe(SO4)2”¤6H2O(M=
(4)»ģŗĻĪļÖŠCu2SµÄÖŹĮæ·ÖŹżĪŖ________”£?
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
8![]() +5Cu2S+44H+
+5Cu2S+44H+![]() 10Cu2++5SO2+8Mn2++22H2O
10Cu2++5SO2+8Mn2++22H2O
6![]() +5CuS+28H+
+5CuS+28H+![]() 5Cu2++5SO2+6Mn2++14H2O
5Cu2++5SO2+6Mn2++14H2O
·“Ó¦ŗóÖó·ŠČÜŅŗ£¬øĻ¾”SO2£¬Ź£ÓąµÄKMnO4Ē”ŗĆÓė350 mL 0.1 mol”¤L-1(NH4)2Fe(SO4)2ČÜŅŗĶźČ«·“Ó¦”£
(1)ÅäĘ½KMnO4Óė(NH4)2Fe(SO4)2·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ
”õ![]() +”õFe2++”õH+
+”õFe2++”õH+![]() ”õMn2++”õFe3++”õH2O
”õMn2++”õFe3++”õH2O
(2)KMnO4ČÜŅŗÓė»ģŗĻĪļ·“Ó¦ŗó£¬Ź£ÓąKMnO4µÄĪļÖŹµÄĮæĪŖ_____________mol”£
(3)ÓūÅäÖĘ500 mL 0.1 mol”¤L-1 Fe2+ČÜŅŗ£¬Šč³ĘČ”(NH4)2Fe(SO4)2”¤6H2O(M=
(4)»ģŗĻĪļÖŠCu2SµÄÖŹĮæ·ÖŹżĪŖ_____________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
2 g Cu2SŗĶCuSµÄ»ģŗĻĪļŌŚĖįŠŌČÜŅŗÖŠÓĆ400 mL 0.075 mol”¤L-1 KMnO4ČÜŅŗ“¦Ąķ£¬·¢Éś·“Ó¦ČēĻĀ£ŗ
8![]() +5Cu2S+44H+====10Cu2++5SO2+8Mn2++22H2O
+5Cu2S+44H+====10Cu2++5SO2+8Mn2++22H2O
 6
6![]() +5CuS+28H+====5Cu2++5SO2+6Mn2++14H2O
+5CuS+28H+====5Cu2++5SO2+6Mn2++14H2O
·“Ó¦ŗóÖó·ŠČÜŅŗ£¬øĻ¾”SO2£¬Ź£ÓąµÄKMnO4Ē”ŗĆÓė350 mL 0.1 mol”¤L-1(NH4)2Fe(SO4)2ČÜŅŗĶźČ«·“Ó¦”£
(1)ÅäĘ½KMnO4Óė(NH4)2Fe(SO4)2·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ
![]()
![]() +
+![]() Fe2++
Fe2++![]() H+
H+![]() Mn2++
Mn2++![]() Fe3++
Fe3++![]() H2O
H2O
(2)KMnO4ČÜŅŗÓė»ģŗĻĪļ·“Ó¦ŗó£¬Ź£ÓąKMnO4µÄĪļÖŹµÄĮæĪŖ_________mol”£
(3)ÓūÅäÖĘ500 mL 0.1 mol”¤L-1 Fe2+ČÜŅŗ£¬Šč³ĘČ”(NH4)2Fe(SO4)2”¤6H2O(M=392 g”¤mol-1)µÄÖŹĮæĪŖ_________ g”£
(4)»ģŗĻĪļÖŠCu2SµÄÖŹĮæ·ÖŹżĪŖ_________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
2 g Cu2SŗĶCuSµÄ»ģŗĻĪļŌŚĖįŠŌČÜŅŗÖŠÓĆ400 mL 0.075 mol”¤L-1 KMnO4ČÜŅŗ“¦Ąķ£¬·¢Éś·“Ó¦ČēĻĀ£ŗ
![]() 8
8![]() +5Cu2S+44H+====10Cu2++5SO2+8Mn2++22H2O
+5Cu2S+44H+====10Cu2++5SO2+8Mn2++22H2O
![]() 6
6![]() +5CuS+28H+====5Cu2++5SO2+6Mn2++14H2O
+5CuS+28H+====5Cu2++5SO2+6Mn2++14H2O
![]() ·“Ó¦ŗóÖó·ŠČÜŅŗ£¬øĻ¾”SO2£¬Ź£ÓąµÄKMnO4Ē”ŗĆÓė350 mL 0.1 mol”¤L-1(NH4)2Fe(SO4)2ČÜŅŗĶźČ«·“Ó¦”£
·“Ó¦ŗóÖó·ŠČÜŅŗ£¬øĻ¾”SO2£¬Ź£ÓąµÄKMnO4Ē”ŗĆÓė350 mL 0.1 mol”¤L-1(NH4)2Fe(SO4)2ČÜŅŗĶźČ«·“Ó¦”£![]()

![]()
![]()
![]() (1)ÅäĘ½KMnO4Óė(NH4)2Fe(SO4)2·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ
(1)ÅäĘ½KMnO4Óė(NH4)2Fe(SO4)2·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ
![]()
![]()
![]() +
+![]() Fe2++
Fe2++![]() H+
H+![]() Mn2++
Mn2++![]() Fe3++
Fe3++![]() H2O
H2O
![]() (2)KMnO4ČÜŅŗÓė»ģŗĻĪļ·“Ó¦ŗó£¬Ź£ÓąKMnO4µÄĪļÖŹµÄĮæĪŖ_________mol”£
(2)KMnO4ČÜŅŗÓė»ģŗĻĪļ·“Ó¦ŗó£¬Ź£ÓąKMnO4µÄĪļÖŹµÄĮæĪŖ_________mol”£
![]() (3)ÓūÅäÖĘ500 mL 0.1 mol”¤L-1 Fe2+ČÜŅŗ£¬Šč³ĘČ”(NH4)2Fe(SO4)2”¤6H2O(M=392 g”¤mol-1)µÄÖŹĮæĪŖ_________ g”£
(3)ÓūÅäÖĘ500 mL 0.1 mol”¤L-1 Fe2+ČÜŅŗ£¬Šč³ĘČ”(NH4)2Fe(SO4)2”¤6H2O(M=392 g”¤mol-1)µÄÖŹĮæĪŖ_________ g”£
![]() (4)»ģŗĻĪļÖŠCu2SµÄÖŹĮæ·ÖŹżĪŖ_________”£
(4)»ģŗĻĪļÖŠCu2SµÄÖŹĮæ·ÖŹżĪŖ_________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2015½ģ¼ŖĮÖŹ”ø߶žÉĻŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
Ć÷·ÆŹÆŹĒÖĘČ”¼Ų·ŹŗĶĒāŃõ»ÆĀĮµÄÖŲŅŖŌĮĻ£¬Ć÷·ÆŹÆµÄ×é³ÉŗĶĆ÷·ÆĻąĖĘ£¬“ĖĶā»¹ŗ¬ÓŠŃõ»ÆĀĮŗĶÉŁĮæŃõ»ÆĢśŌÓÖŹ”£¾ßĢåŹµŃé²½ÖčČēĻĀĶ¼ĖłŹ¾£ŗ
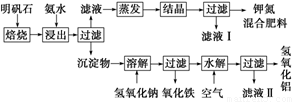
øł¾ŻÉĻŹöĶ¼Ź¾£¬Ķź³ÉĻĀĮŠĢīæÕ£ŗ
£Ø1£©Ć÷·ÆŹÆ±ŗÉÕŗóÓĆĻ”°±Ė®½ž³ö”£ÅäÖĘ500 mLĻ”°±Ė®(ĆæÉżŗ¬ÓŠ39.20 g°±)ŠčŅŖČ”ÅØ°±Ė®(ĆæÉżŗ¬ÓŠ251.28 g°±)__________mL£¬ÓĆ¹ęøńĪŖ__________mLĮæĶ²ĮæČ””£
£Ø2£©°±Ė®½ž³öŗóµĆµ½¹ĢĢå»ģŗĻĢåĻµ£¬¹żĀĖ£¬ĀĖŅŗÖŠ³żK£«”¢SO42-Ķā£¬»¹ÓŠ“óĮæµÄNH4+”£¼ģŃéNH4+µÄ·½·ØŹĒ_________________________________________________________________”£
£Ø3£©Š“³ö³ĮµķĪļÖŠĖłÓŠĪļÖŹµÄ»ÆѧŹ½___________________________________”£
£Ø4£©ĀĖŅŗ¢ńµÄ³É·ÖŹĒĖ®ŗĶ______________________”£
£Ø5£©ĪŖ²ā¶Ø»ģŗĻ·ŹĮĻK2SO4”¢(NH4)2SO4ÖŠ¼ŲµÄŗ¬Į棬ĒėĶźÉĘĻĀĮŠ²½Öč£ŗ
¢Ł³ĘČ”¼ŲµŖ·ŹŹŌŃł²¢ČÜÓŚĖ®£¬¼ÓČė×ćĮæ__________ČÜŅŗ£¬²śÉś°×É«³Įµķ”£
¢Ś__________”¢__________”¢__________(ŅĄ“ĪĢīŠ“ŹµŃé²Ł×÷Ćū³Ę)”£
¢ŪĄäČ“”¢³ĘÖŲ”£
£Ø6£©ČōŹŌŃłĪŖm g£¬³ĮµķµÄĪļÖŹµÄĮæĪŖn mol£¬ŌņŹŌŃłÖŠK2SO4µÄĪļÖŹµÄĮæĪŖ__________mol(ÓĆŗ¬ÓŠm”¢nµÄ“śŹżŹ½±ķŹ¾)”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com