
��1��������һ����Ҫ�Ļ���ԭ�ϡ�Ŀǰ�Ƽҵ��Ҫ�С�������͡������Ƽ���������Ƽ�����ֹ��ա�
�١��������������CaCl2�����д���ù����в���CaCl2�Ļ�ѧ����ʽ��_________________________________________________��
��д���������Ƽ���йط�Ӧ�Ļ�ѧ����ʽ_________________ _�� ��
��CO2���Ƽҵ����Ҫԭ�ϣ��������Ƽ���롰�������CO2����Դ�кβ�ͬ��________________________________________��
��2��������ҵ�Դٽ����ú���ᷢչ������Ҫ���á�
������ʱ������衢�̺�����Ŀ����_______________________________��
�ڲ���ֺ��е�CrԪ���������ֹ��̵�����__ __���ǰ�������롣
�����������������У�β�������е���Ҫ��Ⱦ����________���ӻ����;��ýǶȿ��ǣ�����β��������������_________��
��1����2NH4Cl��Ca(OH)2 2NH3����CaCl2��2H2O ��2�֣�
2NH3����CaCl2��2H2O ��2�֣�
��NH3��CO2��H2O��NaCl�����ͣ���NaHCO3����NH4Cl ��2�֣�
2NaHCO3 Na2CO3��CO2����H2O ��2�֣�
Na2CO3��CO2����H2O ��2�֣�
����д�ܷ�Ӧ����ʽ��2NaCl��2NH3��CO2��H2O��Na2CO3��2NH4Cl��
�ۡ������CO2��Դ��ʯ��ʯ���գ��������Ƽ��CO2��Դ�ںϳɰ���ҵ�ķ�������2�֣�
��2���������͵����ֵijɷ� ��2�֣�
�ں� ��1�֣�
��CO ��2�֣� ȼ�ϣ���ԭ���� ��2�֣�
���������������1�� �ٰ����Ϊ���հ���ʹʯ�����븱�����Ȼ�立�Ӧ���Ӷ���������CaCl2������2NH4Cl + Ca(OH)2=CaCl2+2H2O+2NH3�����������Ƽ����Ҫ��ѧ��ӦΪ��
NaCl(����)+CO2+NH3+H2O=NaHCO3��+NH4Cl 2NaHCO3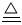 Na2CO3+H2O+CO2��
Na2CO3+H2O+CO2��
�۰����CO2��Դ��ʯ��ʯ�����գ��������Ƽ����CO2��Դ�ںϳɰ���ҵ�ķ�����
��2��������ʱ������衢�̺�����Ҫ�ǿ��������͵����ֵijɷ֣��� ��ΪCr�ױ�������Ϊ��ֹCr������������ֺ��е�CrԪ���������ֹ��̵�������������ǰ����Cr���γ�¯������ȥ�������������������У�CO����Ҫ�Ļ�ԭ������β�������е���Ҫ��Ⱦ����CO��һ����̼�������ж������������д�����
���㣺���黯ѧ�뼼����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ˮ�Ǿ����Դ���⣬�ں�ˮ�������ۺ����÷��棬�����λ��ȫ��ǰ�С��Ӻ�ˮ����ȡʳ�κ���Ĺ������£�
��1�����оٺ�ˮ���������ַ�����________��________��
��2����NaCl��Һ���е�⣬�ڵ����п�ֱ�ӵõ��IJ�Ʒ��H2��________��________��H2��________��
��3����������ѻ��Br2����������ֽ�Br2��ԭΪBr������Ŀ��Ϊ_____________��
��4���������SO2ˮ��Һ����Br2�������ʿɴ�95%���йط�Ӧ�����ӷ���ʽ ���ɴ˷�Ӧ��֪�������������⣬�ڹ�ҵ������Ӧ�������Ҫ������____________��
��5��ij��ѧ�о���ѧϰС��Ϊ�˽�ӹ�ҵ�����ᴿ��ķ������������й����ϣ�Br2�ķе�Ϊ59�棬����ˮ���ж��Ժ�ǿ��ʴ�ԡ����Dzι��������̺���������װ�ü�ͼ��
�������������ۣ�
��ͼ������B�����ƣ�________��
������ʵ��װ�����������Ӿ��������������ܣ���ԭ����___________��
��ʵ��װ�����������ã�Ҫ�ﵽ�ᴿ���Ŀ�ģ���������ο��ƹؼ�������____��
��C��Һ�������ɫΪ________��Ϊ��ȥ�ò������Բ���������Cl2���������м���NaBr��Һ����ַ�Ӧ���ٽ��еķ��������________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ҹ��зḻ����Ȼ����Դ������Ȼ��Ϊԭ�Ϻϳ����ص���Ҫ������ͼ��ʾ(ͼ��ijЩת�����輰������δ�г�):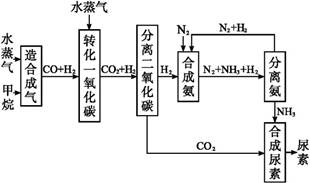
����д���пհ�:
(1)��֪0.5 mol�����0.5 molˮ������t�桢p kPaʱ,��ȫ��Ӧ����һ����̼������(�ϳ���),������a kJ�������÷�Ӧ���Ȼ�ѧ����ʽ����������������
(2)����������,��ҵ�Ϸ���H2��CO2�����ķ�������������
| A���������ͨ������������Һ,������Һ�м������� |
| B���������ѹ��ȴ,ʹCO2Һ�� |
| C��������ð�ˮϴ�� |
| D���������ͨ��ʯ�ҽ���,Ȼ��������չ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���Ų��Ͽ�ѧ�ķ�չ�����������仯����õ���Խ��Խ�㷺��Ӧ�ã�������Ϊ���Ͻ�ά���ء�����ҵ�ϻ��շϷ�����������V2O5��VOSO4��K2SO4��SiO2���з�����Ҫ�������£�
��֪����1��V2O5��NH4VO3��Ϊ�����VOSO4��(VO2)2SO4��Ϊ�����
��2�� 2VO2++H2C2O4+2H+ = 2VO2+ + 2CO2��+ 2H2O
�ش��������⣺
��1�������ǰ�������Ŀ����_________________________��
��2��������з�����Ӧ�����ӷ���ʽΪ__________________________��
��3������۵ı仯���̿ɼ�Ϊ(HA��ʾ�л���ȡ��)��
VOSO4 (ˮ��)+ 2HA���л��㣩 VOA2(�л��㣩+ H2SO4(ˮ��)��������п�ѡ������������ȡ��ԭ����_____________��
VOA2(�л��㣩+ H2SO4(ˮ��)��������п�ѡ������������ȡ��ԭ����_____________��
��4���������ữ��H2C2O4��Һ�ζ�(VO2)2SO4��Һ���Բⶨ�����ݺ���Һ�к������IJ���Ϊ��ȡ10.0mL0.1mol/LH2C2O4��Һ����ƿ�У�����ָ�����������Һʢ���ڵζ����У��ζ����յ�ʱ�����Ĵ���Һ�����Ϊ10.0mL���ɴ˿�֪(VO2)2SO4��Һ��Ԫ�صĺ���Ϊ_________g/L��
��5��V2O5���ý���(��Ca��Al)�Ȼ�ԭ����÷�����������Ȼ�ԭ�Ƶ÷��Ļ�ѧ����ʽΪ_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���������ƣ�Na2S2O5��������ʳƷƯ�������Ʊ������������£�
��֪����Ӧ�����2NaHSO3 Na2S2O5��H2O�ȶಽ��Ӧ��
Na2S2O5��H2O�ȶಽ��Ӧ��
��1��ʵ������ȡ�����Ļ�ѧ����ʽ�� ��
��2�������ա�ʱ������Ӧ�Ļ�ѧ����ʽ�� ��
��3����֪Na2S2O5��ϡ���ᷴӦ�ų�SO2�������ӷ���ʽΪ�� ��
��4������ƷX�Ļ�ѧʽ�ǣ� ����ѭ�����õ������ǣ�_________��_______��
��5��Ϊ�˼��ٲ�ƷNa2S2O5�����ʺ���������Ʒ�Ӧ�����������������ʵ���֮��ԼΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���������ƣ�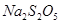 ��������ʳƷƯ�������Ʊ������������£�
��������ʳƷƯ�������Ʊ������������£�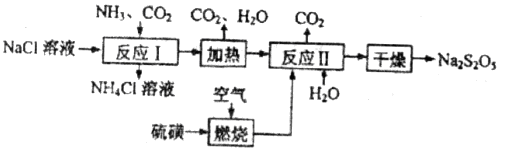
��֪����Ӧ����� �ȶಽ��Ӧ��
�ȶಽ��Ӧ��
��1����Ӧ��Ļ�ѧ����ʽΪ____________����Ӧ�����ʱӦ��ͨ��__________���塣
��2�����ȼ��ǰ�ȼ��ȳ�Һ̬��ͨ�������������¯�У�Ŀ����__________�����������������п�ѭ��ʹ�õ�������_____________��
��3����Ӧ��������Ʋμӷ�Ӧ���������������ʵ���֮�Ƚӽ�____________�������������㣬��ᵼ��_______________��
��4��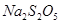 ��ϡ���ᷴӦ�ų�
��ϡ���ᷴӦ�ų�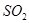 �������ӷ���ʽΪ___________��
�������ӷ���ʽΪ___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����ҹ����Ԫ�أ����۵���ߵĽ������㷺�������Ƶ��ݵĵ�˿���С�����ʹ�ߡ���������������Ȼ����Ҫ���٣�+6�ۣ����ε���ʽ���ڡ��п��ɼ�ֵ���ٿ�ʯ�ǰ��ٿ�ͺ��ٿ��ٿ����Ҫ�ɷ�������ƣ�CaWO4�������ٿ����Ҫ�ɷ��������̵������Σ���ѧʽ��д�ɣ�Fe��Mn��WO4�����ٿ�ͳұ�����յĵ�һ���Ǽ��۷���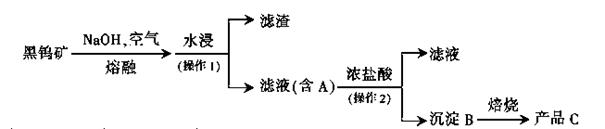
����A��B��C�����ٵĻ�����ش�
��1������ʱ����������ת��Ϊ�������������ƣ�д����Ӧ��Ӧ�Ļ�ѧ����ʽ____��
��2������2�������� ��ʵ������֤������B�Ƿ�ϴ���ķ����� ��ʵ�����б�����Ҫ����Ҫ������____��
��3��д����������ԭ��������ȡ�����ٵĻ�ѧ����ʽ�� ��Ϊ�˻�ÿ������Ƶ�˿�ĸߴ��Ƚ����٣�������̼����������������ԭ������Ϊ____��
��4��ij����ɫ�����ٵĻ�ѧʽ���Ա�ʾΪWO2.8��һ����Ϊ����ɫ�����ٵ���ɫ�ͷ����Ȱ�ʾ���ڻ������д�����ۺ��������ּ�̬���١�����ɫ����������������������ٵ�ԭ����Ŀ֮��Ϊ ��_ ___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�ҹ����������ձ��ȹ��Ҷ������Ƴ�һ���մɲ��ͻ������ֲ��ͻ��ķ���������������������һ�������Ҳ��״��ȵIJ���������ģ����ֲ�����(����)��
| A���������մ� | B���������մ� |
| C�����ά | D�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���й�ҵ�����У������ʵ����ʵ�����Ĺ�ϵʽ����ȷ���ǣ� ��
| A����Ư�ۣ�2Cl2��Ca(ClO)2 |
| B����H2SO4��FeS2��2H2SO4 |
| C���ϳɰ���C��H2��2/3NH3 |
| D����HNO3��NH3��HNO3 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com