
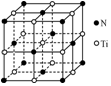
 TiO2��һ�����������İ뵼������������Ч�ؽ��л���Ⱦ����ȩ���ױ��ȣ��ͺ����������NH3��CN-�ȣ�ת��ΪCO2��N2��С�������ʣ�
TiO2��һ�����������İ뵼������������Ч�ؽ��л���Ⱦ����ȩ���ױ��ȣ��ͺ����������NH3��CN-�ȣ�ת��ΪCO2��N2��С�������ʣ����� ��1���˵����=ԭ����������ԭ��������֪�����Ų���Ti��ԭ������Ϊ22��ԭ�Ӻ��������Ϊ22�������������ԭ������������ԭ���ͺ��ع�����д��
��2������Cԭ�����ɦҼ���Ŀȷ���ױ�������̼ԭ�ӹ�����ӻ����ͣ��ӻ������=�ļ���+�¶Ե��Ӷ�����
��3��������ˮ����֮�����γ�������������ڼ��Է��ӣ���������ˮ��Ӧ����һˮ�ϰ����ݴ˷�����
��4�����ݵȵ�������ָ������ͬ������Ŀ��ԭ����Ŀ�ķ��ӻ��������ش�
��5�����������ṹ�жϦҼ�����Ŀ��
��6�����ݾ���Ϊ���������ѻ����㾧���е�ԭ����λ����
��� �⣺��1��Ti��ԭ������Ϊ22��ԭ�Ӻ��������Ϊ22��λ�ڵ������ڵڢ�B�壬������d���ӣ������Ų�Ϊ��1s22s22p63s23p63d24s2����[Ar]3d24s2����
�ʴ�Ϊ��1s22s22p63s23p63d24s2����[Ar]3d24s2����
��2���ױ������м���Cԭ�ӳ�4���Ҽ�������ȡsp3�ӻ���������̼ԭ���ӻ������Ϊ3�����Բ�ȡsp2�ӻ���
�ʴ�Ϊ��sp3��sp2��
��3���������Ǽ��Է��ӣ�ˮ�Ǽ����ܼ�������������ԭ����֪������������ˮ����������ˮ��Ӧ����һˮ�ϰ���ʹ�����ܽ����������ˮ����֮�����γ������ʹ�����ܽ������
�ʴ�Ϊ����������ˮ���ӿ��γɷ��Ӽ������
��4��CNO-�е�����Ϊ��6+7+8+1=22����CNO-������ͬ������Ŀ��ԭ����Ŀ�ǵȵ����壬�������ӻ���CO2����N2O��CS2���ȣ�
�ʴ�Ϊ��N2O��CO2�ȣ�
��5��1molTi��1molCl��5molH2O���γ�6mol�Ҽ���5molH2O�ں���5��2=10mol�Ҽ���������6+10=16mol��16��6.02��1023��
�ʴ�Ϊ��16 mol��16��6.02��1023��
��6����ͼ��֪������Ϊ���������ѻ����Զ����Nԭ�ӷ�����λ�����ĵ�ԭ����֮���ڣ�1������ԭ��Ϊ12���湲�ã�����Tiռ$\frac{1}{2}$������λ��Ϊ6��
�ʴ�Ϊ��6��
���� ���⿼������Ų�ʽ���ӻ����͵��ж��Լ��йؾ���ļ��㣬����жϷ��ӿռ乹���Լ��йؾ������ȷ����Ǹ�Ƶ���㣬Ҳ�Ǹ�����ѵ㣬��Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ��ѧ�� | C-H | O-H | C=O | H-H |
| ����������kJ/mol�� | 414 | 464 | 803 | 436 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��Ȳͨ�����Ը��������Һ�У����������Һ��ɫ | |
| B�� | �ױ���һ����������Ũ���ᷴӦ�����������ױ� | |
| C�� | �Ҵ���һ���������������ᷴӦ���������� | |
| D�� | ��һ�������±���������Ӧ��ȡ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 X��Y��Z��W��Ϊ����������Ԫ�أ�������������Ӧˮ��������£�Ũ�Ⱦ�Ϊ0.1mol•L-1����pH��ԭ�������Ĺ�ϵ��ͼ��ʾ�������й�˵����ȷ����
X��Y��Z��W��Ϊ����������Ԫ�أ�������������Ӧˮ��������£�Ũ�Ⱦ�Ϊ0.1mol•L-1����pH��ԭ�������Ĺ�ϵ��ͼ��ʾ�������й�˵����ȷ����| A�� | �����Ӱ뾶��W��Z��Y��X | B�� | ���⻯���ȶ��ԣ�X��Z��W | ||
| C�� | ����þ�Ͻ�ʱ������X2�������� | D�� | ������Y2Z2�д������Ӽ����ۼ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | H2SO4��Ũ��$\stackrel{Fe}{��}$SO2$\stackrel{BaCl_{2}��aq��}{��}$BaSO3��s�� | |
| B�� | NH3$��_{��������}^{O_{2}}$NO$\stackrel{H_{2}O}{��}$HNO3 | |
| C�� | SiO2$��_{��}^{NaOH��AQ��}$Na2SiO3��aq��$\stackrel{����CO_{2}}{��}$H2SiO3 | |
| D�� | NaAlO2$\stackrel{����}{��}$AlCl3��aq��$\stackrel{��}{��}$AlCl3��s�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| Ԫ�ش��� | X | Y | Z | M | R |
| ԭ�Ӱ뾶/nm | 0.186 | 0.102 | 0.075 | 0.074 | 0.143 |
| ��Ҫ���ϼ� | +1 | +6-2 | +5-3 | -2 | +3 |
 ��������ѧ���������Ӽ������ۼ���
��������ѧ���������Ӽ������ۼ����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
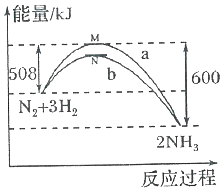 ��һ���¶�ʱ��N2��H2��Ӧ�����������仯��������ͼ������a��ʾ��ʹ�ô���ʱ�������仯���ߣ�b��ʾʹ�ô���ʱ�������仯���ߣ�����������ȷ���ǣ�������
��һ���¶�ʱ��N2��H2��Ӧ�����������仯��������ͼ������a��ʾ��ʹ�ô���ʱ�������仯���ߣ�b��ʾʹ�ô���ʱ�������仯���ߣ�����������ȷ���ǣ�������| A�� | ״̬M��N����ʾ2molN��g��+6mol H��g�� | |
| B�� | �÷�Ӧ���Ȼ�ѧ����ʽΪ��N2+3H2?2NH3��H=-92kJ•mol-l | |
| C�� | ʹ�ô����������˷�Ӧ�����������������������˷�Ӧ�ų������� | |
| D�� | ʹ�ô����������ܸı䷴Ӧ�ġ�H |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���� | B�� | �ױ� | C�� | ClO3- | D�� | SO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
 ����ԭCO2�ǽ������ЧӦ����Դ�������Ҫ�ֶ�֮һ���о���������Cu/ZnO���������£�CO2��H2�ɷ���������Ӧ���ֱ�����CH3OH��CO����Ӧ���Ȼ�ѧ����ʽ���£�CO2��g��+3H2��g��?CH3OH��g��+H2O��g����H1=-53.7kJ•mol-1 I��CO2��g��+H2��g��?CO��g��+H2O��g����H2��
����ԭCO2�ǽ������ЧӦ����Դ�������Ҫ�ֶ�֮һ���о���������Cu/ZnO���������£�CO2��H2�ɷ���������Ӧ���ֱ�����CH3OH��CO����Ӧ���Ȼ�ѧ����ʽ���£�CO2��g��+3H2��g��?CH3OH��g��+H2O��g����H1=-53.7kJ•mol-1 I��CO2��g��+H2��g��?CO��g��+H2O��g����H2��| T��K�� | ���� | CO2ת���ʣ�%�� | �״�ѡ���ԣ�%�� |
| 543 | Cat.1 | 12.3 | 42.3 |
| 543 | Cat.2 | 10.9 | 72.7 |
| 553 | Cat.1 | 15.3 | 39.1 |
| 553 | Cat.2 | 12.0 | 71.6 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com