
 ����δ�������ų�A
����δ�������ų�A


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
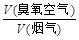 ������Ϊy����ͨ�������г�y��x�Ĺ�ϵʽ��
������Ϊy����ͨ�������г�y��x�Ĺ�ϵʽ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Ư���� | B�������� | C����ԭ�� | D����������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ҵ�����г�����ʯ�������շ����еĶ������� |
| B�����������ˮ��Һ��ʹ��ɫʯ����Һ��죬˵��������������ˮ��Ӧ������ |
| C������������ʹ��ɫ������ɫ��ʧ��˵������������������� |
| D������������ʹ���Ը��������Һ��ɫ��˵������������л�ԭ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��CaO | B��Na2CO3 | C��Ca(OH)2 | D��CaCO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
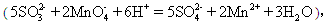 ����KMnO4��Һb mL��
����KMnO4��Һb mL���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��һ���� CO32- | B��һ����Ag+ | C��һ����SO42- | D�����϶� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��n =" 2" | B��SCln��ÿ��ԭ������������8�����ȶ��ṹ |
| C��SCln�ڹ�̬ʱΪ���Ӿ��� | D��SCln����ˮ�ܵ����Cl-- |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com