
³¬ĻøŃõ»ÆĀĮŹĒŅ»ÖÖÖŲŅŖµÄ¹¦ÄÜĢÕ“ÉŌĮĻ”£
£Ø1£©ŹµŃéŹŅ³£ŅŌNH4Al£ØSO4£©2ŗĶNH4HCO3ĪŖŌĮĻ£¬ŌŚŅ»¶ØĢõ¼žĻĀĻČ·“Ӧɜ³É³ĮµķNH4AlO£ØOH£©HCO3£¬øĆ³ĮµķøßĪĀ·Ö½ā¼“µĆ³¬ĻøAl2O3”£NH4AlO£ØOH£©HCO3ČČ·Ö½āµÄ»Æѧ·“Ó¦·½³ĢŹ½ĪŖ_________________________”£
£Ø2£©NH4Al£ØSO4£©2”¤12H2OµÄĻą¶Ō·Ö×ÓÖŹĮæĪŖ453”£ÓūÅäÖĘ100 mL pHĪŖ2”¢ÅضČŌ¼ĪŖ0.1 mol”¤L-1µÄNH4Al£ØSO4£©2ČÜŅŗ£¬ÅäÖĘ¹ż³ĢĪŖ£ŗ
¢ŁÓĆĶŠÅĢĢģĘ½³ĘĮæNH4Al£ØSO4£©2”¤12H2O¹ĢĢå______________________g£»
¢Ś½«ÉĻŹö¹ĢĢåÖĆÓŚÉÕ±ÖŠ£¬_________________________”£
£Ø3£©ŌŚ0.1 mol”¤L-1 NH4Al£ØSO4£©2ČÜŅŗÖŠ£¬ĀĮø÷ŠĪĢ¬µÄÅØ¶Č£ØŅŌAl3+¼Ę£©µÄ¶ŌŹż£Ølgc£©ĖęČÜŅŗpH±ä»ÆµÄ¹ŲĻµ¼ūĻĀĶ¼£ŗ
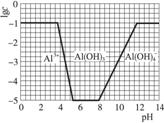
¢ŁÓĆNaOHČÜŅŗµ÷½Ś£Ø2£©ÖŠČÜŅŗpHÖĮ7£¬øĆ¹ż³ĢÖŠ·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ÓŠ___________”£
¢ŚĒėŌŚ“šĢāæصÄæņĶ¼ÖŠ£¬»³ö0.01 mol”¤L-1 NH4Al£ØSO4£©2ČÜŅŗÖŠĀĮø÷ŠĪĢ¬µÄÅØ¶ČµÄ¶ŌŹżlgcĖęČÜŅŗpH±ä»ÆµÄ¹ŲĻµĶ¼£¬²¢½ųŠŠ±ŲŅŖ±ź×¢”£
”¾“š°ø”æ
£Ø1£©2NH4AlO£ØOH£©HCO3 Al2O3+2CO2”ü+2NH3”ü+3H2O”ü
Al2O3+2CO2ӟ+2NH3ӟ+3H2Oӟ
£Ø2£©¢Ł4.5 ¢Ś¼ÓŹŹĮæĻ”ĮņĖįČܽā£¬ŌŁ¼ÓĖ®Ļ”ŹĶÖĮ100 mL
£Ø3£©¢ŁAl3++3OH-====Al(OH)3”ż H++OH-====H2O  +OH-====NH3”¤H2O
+OH-====NH3”¤H2O
¢Ś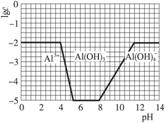
”¾½āĪö”æ(2)¢Łm£½0.1 mol”¤L-1”Į0.1 L”Į453 g”¤mol-1£½4.5 g
¢ŚŅņÅäÖĘČÜŅŗµÄpHĪŖ2£¬ĖłŅŌÅäÖĘČÜŅŗŹ±ŅŖ¼ÓČėŹŹĮæµÄĻ”ĮņĖį”£
£Ø3£©¢ŁpH£½7Ź±£¬Al£ØOH£©3²»Čܽā£¬Ö»ÓŠŅ»øöĄė×Ó·“Ó¦·¢Éś”£
¢Ś0.01 mol”¤L-1 NH4Al£ØSO4£©2ČÜŅŗµÄlgc£½-2”£


 ŠĀæĪ±ź½×ĢŻŌĶĮѵĮ·ĻµĮŠ“š°ø
ŠĀæĪ±ź½×ĢŻŌĶĮѵĮ·ĻµĮŠ“š°ø æŚĖćŠÄĖćĖŁĖćÓ¦ÓĆĢāĻµĮŠ“š°ø
æŚĖćŠÄĖćĖŁĖćÓ¦ÓĆĢāĻµĮŠ“š°ø Ķ¬²½ĶŲÕ¹ŌĶĮĻµĮŠ“š°ø
Ķ¬²½ĶŲÕ¹ŌĶĮĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
FeSO4ŹĒŅ»ÖÖ¾«Ļø»Æ¹¤²śĘ·£¬æÉÓĆÓŚÖĪĮĘȱĢśŠŌʶŃŖ”¢ÖĘøļ”¢Ä¾²Ä·ĄøÆµČ”£Öʱø²½Öč£ŗ¢Ł½«3mol”¤L-1ĮņĖį¼ÓČėĢś·ŪĄļ£¬Ī¢ČČ£¬½Į°čŹ¹Ęä³ä·Ö·“Ó¦£»¢Ś³ĆČČ¹żĀĖ£»¢ŪŌŚ50”ę×óÓŅÕō·¢”¢½į¾§£¬µĆµ½¾§Ģå”Ŗ”ŖĀĢ·Æ£ØFeSO4”¤7H2O£©”£»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ÖʱøFeSO4ČÜŅŗŹ±Ņ»°ć²»ÓĆ½ĻÅØĮņĖį¶ųÓĆ3mol”¤L-1ĮņĖįµÄŌŅņŹĒ___________
________________________________________________________________,·“Ó¦Ź±ŅŖĒóĢś·Ū¹żĮæµÄĄķÓÉŹĒ____________________________________________”£
£Ø2£©¼ģŃé²½Öč¢ŁĖłµĆČÜŅŗÖŠ½šŹōŃōĄė×ӵķ½·ØŹĒ__________________________
_________________________________________________ӣ
£Ø3£©²½Öč¢Ś³ĆČČ¹żĀĖµÄŌŅņŹĒ___________________________________”£
£Ø4£©ŌŚæÕĘųÖŠ¼ÓČČĀĢ·Æ£¬¹ĢĢåÖŹĮæÓėĪĀ¶Č±ä»ÆĒśĻßČēĻĀĶ¼£ŗ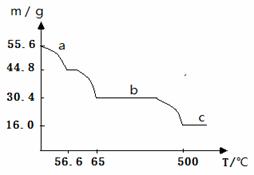
aĒśĻ߶ŌÓ¦µÄ·“Ó¦»Æѧ·½³ĢŹ½ĪŖ___________________________________”£
cĒśĻ߶ŌÓ¦µÄĪļÖŹ»ÆѧŹ½ĪŖ____________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
øßĢśĖį¼Ų( K2FeO4)ŹĒŅ»ÖÖŠĀŠĶµÄ×ŌĄ“Ė®“¦Ąķ¼Į£¬ĖüµÄŠŌÖŹŗĶ×÷ÓĆŹĒ
A£®ÓŠĒæŃõ»ÆŠŌ£¬æÉĻū¶¾É±¾ś£¬»¹Ō²śĪļÄÜĪüø½Ė®ÖŠŌÓÖŹ
B£®ÓŠĒ滹ŌŠŌ£¬æÉĻū¶¾É±¾ś£¬Ńõ»Æ²śĪļÄÜĪüø½Ė®ÖŠŌÓÖŹ
C£®ÓŠĒæŃõ»ÆŠŌ£¬ÄÜĪüø½Ė®ÖŠŌÓÖŹ£¬»¹Ō²śĪļÄÜĻū¶¾É±¾ś
D£®ÓŠĒ滹ŌŠŌ£¬ÄÜĪüø½Ė®ÖŠŌÓÖŹ£¬Ńõ»Æ²śĪļÄÜĻū¶¾É±¾ś
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ÓŠA”¢B”¢C”¢D”¢EŗĶFĮłĘæĪŽÉ«ČÜŅŗ£¬ĖūĆĒŹĒ֊ѧ»Æѧ֊³£ÓƵÄĪŽ»śŹŌ¼Į”£“æEĪŖĪŽÉ«ÓĶדŅŗĢ壻B”¢C”¢DŗĶFŹĒŃĪČÜŅŗ£¬ĒŅĖūĆĒµÄŅõĄė×Ó¾ł²»Ķ¬”£ĻÖ½ųŠŠČēĻĀŹµŃé£ŗ
¢ŁAÓŠ“Ģ¼¤ŠŌĘųĪ¶£¬ÓĆÕ“ÓŠÅØŃĪĖįµÄ²£Į§°ō½Ó½üAŹ±²śÉś°×É«ŃĢĪķ£»
¢Ś½«A·Ö±š¼ÓČėĘäĖüĪåÖŠČÜŅŗÖŠ£¬Ö»ÓŠD”¢FÖŠÓŠ³Įµķ²śÉś£»¼ĢŠų¼ÓČė¹żĮæAŹ±£¬DÖŠ³ĮµķĪŽ±ä»Æ£¬FÖŠ³ĮµķĶźČ«Čܽā£»
¢Ū½«B·Ö±š¼ÓČėC”¢D”¢E”¢FÖŠ£¬C”¢D”¢FÖŠ²śÉś³Įµķ£¬EÖŠÓŠĪŽÉ«”¢ĪŽĪ¶ĘųĢåŅŻ³ö£»
¢Ü½«C·Ö±š¼ÓČėD”¢E”¢FÖŠ£¬¾łÓŠ³ĮµķÉś³É£¬ŌŁ¼ÓČėĻ”HNO3£¬³Įµķ¾ł²»ČÜ”£
øł¾ŻÉĻŹöŹµŃéŠÅĻ¢£¬Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£© ÄÜČ·¶ØČÜŅŗŹĒ£ØŠ“³öČÜŅŗ±źŗÅÓėĻąÓ¦ČÜÖŹµÄ»ÆѧŹ½£©£ŗ
£Ø2£© ²»ÄÜČ·¶ØµÄČÜŅŗ£¬Š“³öĘ䱟ŗÅ”¢ČÜÖŹæÉÄܵĻÆѧŹ½¼°½ųŅ»²½¼ų±šµÄ·½·Ø£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠÓŠ¹Ų½šŹō¼°ĘäŗĻ½šµÄĖµ·Ø²»ÕżČ·µÄŹĒ£Ø £©
A.ÄæĒ°ĪŅ¹śĮ÷ĶصÄÓ²±ŅŹĒÓÉŗĻ½š²ÄĮĻÖĘŌģµÄ
B.ÉśĢś”¢ĘÕĶØøÖŗĶ²»ŠāøÖÖŠµÄĢ¼ŗ¬ĮæŅĄ“ĪŌö¼Ó
C.Ć¾ŌŚæÕĘųÖŠČ¼ÉÕ·¢³öŅ«ŃŪµÄ°×¹ā£¬æÉÓĆÓŚÖĘ×÷ÕÕĆ÷µÆ
D.ČÕÓĆĀĮÖĘĘ·±ķĆęø²øĒ×ÅŃõ»ÆĤ£¬¶ŌÄŚ²æ½šŹōĘš±£»¤×÷ÓĆ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŹµŃéŹŅ½«9 gĀĮ·ŪøśŅ»¶ØĮæµÄ½šŹōŃõ»ÆĪļ·ŪÄ©»ģŗĻŠĪ³ÉĀĮČČ¼Į”£·¢ÉśĀĮČČ·“Ó¦Ö®ŗó£¬ĖłµĆ¹ĢĢåÖŠŗ¬½šŹōµ„ÖŹ18 g£¬ŌņøĆŃõ»ÆĪļ·ŪÄ©æÉÄÜŹĒ( )
A.Fe2O3ŗĶMnO2 B.MnO2ŗĶV2O5
C.Cr2O3ŗĶV2O5 D.Fe3O4ŗĶFeO
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
1 mol¹żŃõ»ÆÄĘÓė2 molĢ¼ĖįĒāÄĘ¹ĢĢå»ģŗĻŗó£¬ŌŚĆܱÕČŻĘ÷ÖŠ¼ÓČČ³ä·Ö·“Ó¦£¬ÅųöĘųĢåĪļÖŹŗóĄäČ“£¬²ŠĮōµÄ¹ĢĢåĪļÖŹŹĒ
A.Na2CO3 B.Na2O2 Na2CO3
C.NaOH Na2CO3 D.Na2O2 NaOH Na2CO3
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŌŚŅ»¶ØĢõ¼žĻĀ£¬½«ÄĘÓėŃõĘų·“Ó¦µÄÉś³ÉĪļ1.5g ČÜÓŚĖ®£¬ĖłµĆČÜŅŗĒ”ŗĆÄܱ»80mLÅضČĪŖ0.50mol/LµÄHClČÜŅŗÖŠŗĶ£¬ŌņøĆÉś³ÉĪļµÄ³É·ÖŹĒ
A.Na2O B.Na2O2
C.Na2OŗĶNa2O2 D.Na2O2ŗĶNaO2
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠŠšŹöÕżČ·µÄŹĒ£Ø””””£©
| ”” | A£® | NaClČÜŅŗŌŚµēĮ÷×÷ÓĆĻĀµēĄė³ÉNa+ÓėCl﹣ |
| ”” | B£® | ČÜÓŚĖ®ŗóÄܵēĄė³öH+µÄ»ÆŗĻĪļ¶¼ŹĒĖį |
| ”” | C£® | ŅŗĢ¬ĀČ»ÆĒā²»Äܵ¼µē£¬µ«ĀČ»ÆĒāŹĒµē½āÖŹ |
| ”” | D£® | µ¼µēŠŌĒæµÄČÜŅŗĄļ×ŌÓÉŅĘ¶ÆĄė×ÓŹżÄæŅ»¶Ø±Čµ¼µēŠŌČõµÄČÜŅŗĄļ×ŌÓÉŅĘ¶ÆĄė×ÓŹżÄæ¶ą |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com