
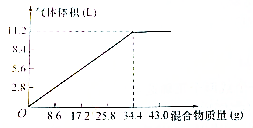
 ��100mL NaOH��Һ�м���NH4NO3�ͣ�NH4��2SO4�̶�����ﹲ�ȣ�����������������Ͳ��������������ѻ���ɱ�װ���Ĺ�ϵ��ͼ��ʾ���ٶ����ɵ�NH3ȫ���ݳ������Լ��㣺
��100mL NaOH��Һ�м���NH4NO3�ͣ�NH4��2SO4�̶�����ﹲ�ȣ�����������������Ͳ��������������ѻ���ɱ�װ���Ĺ�ϵ��ͼ��ʾ���ٶ����ɵ�NH3ȫ���ݳ������Լ��㣺���� ��1������������NH4NO3�ͣ�NH4��2SO4�Ļ�������ɰ�����������ӦNH4++OH-$\frac{\underline{\;\;��\;\;}}{\;}$NH3��+2H2O����ͼ���֪���������34.4gʱ���ɰ����������Ϊ11.2L������21.5�˹�������ʱ���������Ȼ������г�����ʽ��⣻
��2����Ϸ���ʽ���������������ʵ������ٸ���c=$\frac{n}{V}$�����������Ƶ����ʵ���Ũ�ȣ�
��3���������李�����淋����ʵ�����Ȼ����ݶ��ߵ����������ɰ��������ʵ�����ʽ���������李�����淋����ʵ������ɣ�
��4�������������������51.6 gʱ������������ȫ��Ӧ���ĵ�����������Һ�����Ϊ��$\frac{51.6g}{34.4g}$��100ml=150ml����V��NaOH��=140 mL��˵�������������n��NH3��=n��NaOH�����ݴ˼�����
��� �⣺��1���������34.4gʱ���ɰ����������Ϊ11.2L�����Լ���21.5�˹�������ʱ����������������������Ϊ��V���� $\frac{34.4g}{21.5g}=\frac{11.2L}{V}$����֮�ã�V=7L��
��21.5�˹�������ڱ�״���²������������Ϊ7L��
��2����ͼ��֪��100 mL NaOH��Һ��34.4 g ���ǡ����ȫ��Ӧ�����ݣ�NH4++OH-$\frac{\underline{\;\;��\;\;}}{\;}$NH3��+H2O��c��NaOH��=$\frac{\frac{11.2L}{22.4L/mol}}{0.1L}$=5mol/L��
��NaOH��Һ�����ʵ���Ũ��Ϊ5mol/L��
��3����34.4g������NH4NO3�ͣ�NH4��2SO4�����ʵ����ֱ�Ϊxmol��ymol��������������ɵã���80g/mol��x+132/mol��y=34.4 g��
�ٸ������ɰ��������ʵ����ɵã���x+2y=$\frac{11.2}{22.4}$mol��
�٢�������ã�x=0.1��y=0.2���������ߵ����ʵ���֮��Ϊ��1��2��
�𣺹��������У�NH4NO3�루NH4��2SO4�����ʵ���֮��Ϊ1��2��
��4�������������������51.6 gʱ������������ȫ��Ӧ���ĵ�����������Һ�����Ϊ��$\frac{51.6g}{34.4g}$��100ml=150ml����V��NaOH��=140 mL��˵���������������n��NH3��=n��NaOH��=5 mol/L��0.14 L=0.7mol��V��NH3��=0.7mol��22.4 L/mol=15.68 L��
�𣺵�NaOH��Һ�����Ϊ140mL���������������Ϊ51.6g����ַ�Ӧ�����ɵ��������15.68��
���� ������ͼ����ʽ������������йؼ��㣬ע�ⷢ����Ӧ�ı��ʣ��������ӷ���ʽ���н���ۺϿ���ѧ���������������������Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��һ����B��C��ɵĻ�������AC2��Ϊ�ȵ����壬�仯ѧʽΪN2O��
��һ����B��C��ɵĻ�������AC2��Ϊ�ȵ����壬�仯ѧʽΪN2O���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Fe3O4����д��FeO•Fe2O3��Pb3O4Ҳ����д��PbO•Pb2O3 | |
| B�� | �����õ��AlCl3��Һ����ȡ��������Ҳ�����õ��MgCl2��Һ����ȡ����þ | |
| C�� | Fe��Sֱ�ӻ��ϲ��ܵõ�Fe2S3��Al��Sֱ�ӻ���Ҳ���ܵõ�Al2S3 | |
| D�� | FeS���Ժ�ϡ���ᷴӦ��ȡ�������壬CuSҲ���Ժ�ϡ���ᷴӦ��ȡ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���Ƽ����Ҵ��к�������ˮ | |
| B�� | ��������������ͭ��Ӧ������һ������ȩ | |
| C�� | ����������������Ϊͬ���칹�� | |
| D�� | 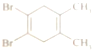 ��ͼ��ʾ�л�����Ӻ˴Ź��������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �������ӣ�����Ũ��ˮ����Һ | |
| B�� | ���ᣨ����þ�������ȣ����� | |
| C�� | ����Һ�����ͣ�����ʳ�ο��������� | |
| D�� | �������������ᣩ��������������Һ����Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��һ����������������������Cu��OH��2������Ӧ | |
| B�� | ��������Ҫ�ɷ�����֬�ڼ���������ˮ�����ɵ� | |
| C�� | ���ۡ���ά�غ���֬������Ȼ�߷��ӻ����� | |
| D�� | ��������Һ������ͭ������ij���������������ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 3��1 | B�� | 1��3 | C�� | 4��1 | D�� | 1��4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ˮ�������������� | |
| B�� | ���ۡ���ά������ˮ������Ϊ���� | |
| C�� | ����ǿ��������ˮ�����ɶ�Ӧ����ʹ� | |
| D�� | ��������NaOH�Ĵ���Һ��ˮ��������ϩ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ۢݢ�� | B�� | �ޢ�� | C�� | �ۢݢ� | D�� | �ۢݢޢߢ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com