[��ѧ--��ѧ�뼼��]���Ṥҵ�ڹ�����ռ�м�����Ҫ�ĵ�λ����ش����Ṥҵ�е��������⣺
��1�����������ĸ�������ѡ��һ���½�һ�����᳧������Ϊ��ַ��ѡ��
C
C
�Ľ��������ţ���
A���зḻ��������Դ�ij���
B����������������
C��������������Ĺ�ҵ����
D���˿ڳ��ܵ��Ļ�����ҵ���ij���
��2��CuFeS
2�ǻ��������һ�ɷ֣�����ʱCuFeS
2ת��ΪCuO��Fe
2O
3 ��SO
2���÷�Ӧ�Ļ�ѧ����ʽΪ
4CuFeS
2+13O
2 4CuO+2Fe
2O
3+8SO
24CuFeS
2+13O
2 4CuO+2Fe
2O
3+8SO
2��
��3�����SO
3�����ʣ�ʵ��������ͨ����
98.3%��Ũ����
98.3%��Ũ����
����SO
3��
��4����֪��Ӧ2SO
2+O
2?2SO
3��H��0���ֽ�0.050mol SO
2��0.030mol O
2�����ݻ�Ϊ1L���ܱ������У���Ӧ��һ�������´ﵽƽ�⣬��÷�Ӧ������ѹǿ��С��ԭ��ѹǿ��75%�����������SO
2��ת����Ϊ
80%
80%
���������µ�ƽ�ⳣ��Ϊ
1.6��103
1.6��103
��
��5�������᳧����¯�ų��Ŀ����к���Fe
2O
3��CuO��CuSO
4����CuO��SO
3 �ڷ���¯�л��϶��ɣ�����������ͭ���������������¯�¶Ȳ�ͬ���仯�����±���
| ����¯�¶�/�� |
600 |
620 |
640 |
660 |
| ¯����CuSO4����������/% |
9.3 |
9.2 |
9.0 |
8.4 |
��֪CuSO
4 �ڵ���660��ʱ����ֽ⣬���Ҫ�����ϱ���CuSO
4�������������¶����߶����͵�ԭ��
SO2ת��ΪSO3������Ӧ���ȵĿ��淴Ӧ�����¶����ߣ�ƽ�����ƣ�SO3���ʵ������٣�����CuSO4��������
SO2ת��ΪSO3������Ӧ���ȵĿ��淴Ӧ�����¶����ߣ�ƽ�����ƣ�SO3���ʵ������٣�����CuSO4��������
��
��6�������Ṥҵβ���У�SO
2����Ҫ������Ⱦ�������о���������������������
ʯ��ˮ
ʯ��ˮ
�������ƣ����գ�Ȼ���������ᴦ������������SO
2��һ������ˮ��ĸ��ϣ�д����������Ӧ�Ļ�ѧ����ʽ
SO2+Ca��OH��2�TCaSO3��+H2O��CaSO3+H2SO4�TCaSO4+SO2��+H2O��
SO2+Ca��OH��2�TCaSO3��+H2O��CaSO3+H2SO4�TCaSO4+SO2��+H2O��
��

![]() 2SO3(g) ����H����196.6kJ?mol��1�����º����´ﵽƽ��ʱ���������A�зų�3.932 kJ����������
2SO3(g) ����H����196.6kJ?mol��1�����º����´ﵽƽ��ʱ���������A�зų�3.932 kJ����������![]() 2SO3(g)��ƽ�ⳣ��K= ��
2SO3(g)��ƽ�ⳣ��K= ��
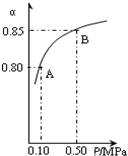 ��������Ҫ�ɷ�ΪFeS2���ǹ�ҵ��ȡ�������Ҫԭ�ϣ������ղ���ΪSO2��Fe2O3��
��������Ҫ�ɷ�ΪFeS2���ǹ�ҵ��ȡ�������Ҫԭ�ϣ������ղ���ΪSO2��Fe2O3��