
ij�о���ѧϰС��������롰�о�����ˮ��Ӧ���ù������ʵijɷ֡����ʼ������á�ʵ��̽��������ͬ����������⣺

��̽��һ�������ͼ��ʾװ�ý��С�����ˮ��Ӧ����ʵ�顣
��1��Ӳ�ʲ������з�����Ӧ�Ļ�ѧ����ʽΪ______________________________��
��2����ӦǰA��Ͷ�����Ƭ��Ŀ����____________________________________��
��3��װ��E�е�������________________________________________________��
��̽�������������ʵ�鷽��ȷ����Ӧ��Ӳ�ʲ������к�ɫ����ijɷ֡�
��4����Ӳ�ʲ�����B��ȴ��ȡ�������еĹ�����������_______��������Һ�ֳ����ݡ�
��5��һ�ݵμӼ���KSCN��Һ������Һ���ɫ���ƶ�Ӳ�ʲ�����B�й������ʵijɷ֣�ѡ����ţ���ͬ��Ϊ_______������Һδ���ɫ���ƶ�Ӳ�ʲ�����B�й������ʵijɷ�Ϊ_______��
��һ����Fe3O4 ��һ����Fe
��ֻ��Fe3O4 ��ֻ��Fe
��6����һ����_______�����������ƣ�����_______������֤����Һ�д���Fe2+��
��̽����������������̲ⶨ��Ӧ��Ӳ�ʲ�����B�й��庬��Ԫ�ص�����������

��7���Լ�b�Ļ�ѧʽ��_______��
��8�����㷴Ӧ��Bװ������Ԫ�ص���������Ϊ_______��
��1��


��2����ֹ����
��3����ɫ�����죬�Ҷ˹ܱ���ˮ��
��4��ϡ���� ��5���� ��
��6����ͷ�ι� ����KMnO4��Һ����Һ��ɫ
��7��NaOH ��8��77.8%
����������̽��һ����1��Fe��ˮ��Ӧ�Ļ�ѧ����ʽΪ��
3Fe+4H2O��g�� Fe3O4+4H2��
Fe3O4+4H2��
��2�����Ƭ�������Ƿ�ֹ���С�
��3��װ��E�з�����Ӧ��H2+CuO Cu+H2O�������ǣ���ɫ�����죬�Ҷ˹ܱ���ˮ�顣
Cu+H2O�������ǣ���ɫ�����죬�Ҷ˹ܱ���ˮ�顣
��̽����������֤��Ӧ���ɫ����ijɷ�ʱ������Fe3+������Լ�ΪKSCN��Һ����ȷ������Fe3+ʱ������Fe2+����������KMnO4��Һ����������KMnO4��Һ�����ᷢ����Ӧ���������ܽⷴӦ��ĺ�ɫ����ʱ�����������ᣬҲ���������ᣨ��ΪHNO3������Fe2+��������ϡ���ᡣ
��̽�������ɡ�����ɫ���塱֪��������ΪFe2O3���� ��NaCl������
��NaCl������ �������Լ�bΪNaOH��Һ��m��Fe2O3��=32 g,��n��Fe2O3��=0.2 mol,��n��Fe��=0.4 mol,��Ӧ��Bװ������Ԫ�ص���������Ϊ��
�������Լ�bΪNaOH��Һ��m��Fe2O3��=32 g,��n��Fe2O3��=0.2 mol,��n��Fe��=0.4 mol,��Ӧ��Bװ������Ԫ�ص���������Ϊ�� ��100%��77.8%��
��100%��77.8%��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2014�껯ѧ�߿��ܸ�ϰ��ʱ���� 5-2Ԫ�����ڱ� Ԫ����������ϰ���������棩 ���ͣ�ѡ����
Ԫ�ص�ԭ�ӽṹ���������ʺ����ڱ��е�λ�á�����˵����ȷ���ǣ� ��
A��Ԫ��ԭ�ӵ���������������Ԫ�ص�����ϼ�
B�������ԭ���У�����˽Ͻ����������˶��ĵ��������ϸ�
C��P��S��Cl�õ�������������������Ӧ��ˮ��������Ծ�������ǿ
D��Ԫ�����ڱ���λ�ڽ����ͷǽ����ķֽ��߸�����Ԫ�����ڹ���Ԫ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�껯ѧ�߿��ܸ�ϰ��ʱ���� 4-3������Ҫ��������ϰ���������棩 ���ͣ�ѡ����
SO2ͨ��BaCl2��Һ�в���������������ͨ����һ���������Բ�����ɫ��������ͼ���Ҳ�Y�ι��з��õ�ҩƷ��ϲ�����Ҫ����ǣ���Ҫʱ���Լ��ȣ��� ��
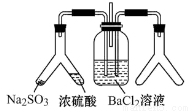
A.Cu��Ũ����
B.CaO��Ũ��ˮ
C.����ʯ��ϡ����
D.���������Һ��Ũ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�껯ѧ�߿��ܸ�ϰ��ʱ���� 4-1���ǽ������ϵ�����-����ϰ���������棩 ���ͣ������
CO2��Ŀǰ�����к�����ߵ�һ���������塣��ˣ����ƺ�����CO2�ǽ������ЧӦ����Ч;����
��1�����й���CO2��˵����ȷ���ǣ�����ţ�_________��
�ټ��ٻ�ʯȼ�ϵ�ʹ�ã��������̫���ܡ����ܵ������Դ����Ч���ʹ�����CO2�ĺ���
��ֲ�����֣�����ֲ�����������Ч���ʹ�����CO2�ĺ���
�۶������������������̼���������ĺ������ǿ��������ձ�������
�ܿ�����CO2�ĺ������ᵼ��������γ�
��2�����д�ʩ���ܼ��ٶ�����̼�ŷŵ��ǣ�����ţ� _________��
������̫��������
�ڹ�ͣС�����ҵ
�۾��С�����һСʱ��Ϩ�ƻ
���ƹ�ʹ��úҺ������
��3�����з�Ӧ����������������ǣ�����ţ�_________��
���ô����Ʋ��� ����ú̿��ȼ��
��������ʯ���� ���ð���̼���
��4��Ŀǰ�����ڶ�����̼�Ƿ�Ϊ������Ⱦ���в�ͬ�Ĺ۵㡣��Ϊ��������̼���Ǵ�����Ⱦ��������ǣ�����ţ�_________��
�ٶ�����̼����Ҫ�Ļ���ԭ��
�ڶ�����̼��ֲ�������õıر�ԭ��
�۶�����̼����ɫ����ζ����������
�ܳ�������̼���⣬���顢һ����������Ҳ����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�껯ѧ�߿��ܸ�ϰ��ʱ���� 4-1���ǽ������ϵ�����-����ϰ���������棩 ���ͣ�ѡ����
�����й�˵����ȷ���ǣ� ��
A. CO2��CH4��N2�Ⱦ����������ЧӦ������
B. 14C���������������14C��12C��Ϊͬ��������
C.�������費���κ��ᷴӦ������ʯӢ������������
D.��������ʱҪ������������ʯӢɰ������ʯӢɰ��Ӧ�ķ���ʽΪ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�껯ѧ�߿��ܸ�ϰ��ʱ���� 3-3��������Ҫ��������ϰ���������棩 ���ͣ�ѡ����
ͨ����ʵ������Ĺ۲졢���������ó���ȷ�Ľ����ǻ�ѧѧϰ�ķ���֮һ��������ʵ����ʵ�Ľ�����ȷ���ǣ� ��
��������������
A��KI-������Һ�м���FeCl3��Һ����Һ����Fe3+������۷�����ɫ��Ӧ
B�����������ڳ�ʪ�Ŀ����У���������һ�����ɫ�İߵ����ڳ�ʪ�Ŀ�����������Fe��OH��3
C��ϡ�����м����������ۣ������ݲ���˵��Fe�û��������е��⣬����������
D����Fe��OH��2¶���ڿ�����һ��ʱ�䣬��ɫ���ʱ���˺��ɫ˵��Fe��OH��2�ױ�O2������Fe��OH��3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�껯ѧ�߿��ܸ�ϰ��ʱ���� 3-2��������Ҫ��������ϰ���������棩 ���ͣ������
��֪A��K��Ϊ��ѧ��ѧ�еij������ʣ�����֮���ת����ϵ��ͼ��ʾ������A��DΪ�������ʣ���Ӧ���������ɵ�ˮ���������ֲ�������ȥ��

��ش��������⣺
��1��A��B��Ӧ�Ļ�ѧ����ʽ�ǣ�______________________________________��
��2����F��ͨ������CO2����K�����ӷ���ʽΪ��_________________________��
��3��A��NaOH��Һ��Ӧ�����ӷ���ʽ�ǣ�_________________________________________________��
��4���ټ�������J����Һ�н������ӵķ����ǣ�_______________________________________________��
�ڽ�����E����ˮ������Һ�����ԣ�ԭ���ǣ������ӷ���ʽ��ʾ��____________��
��ij��Ч��ˮ������D��OH��SO4�ۺϵõ��ġ���ҵ����DSO4��ϡ�������������Ϊԭ�����Ʊ�D��OH��SO4����Ӧ����NO���ɣ��÷�Ӧ�Ļ�ѧ����ʽ�ǣ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�껯ѧ�߿��ܸ�ϰ��ʱ���� 3-1�Ƽ�����Ҫ��������ϰ���������棩 ���ͣ�ѡ����
��2��1 g CO��H2��ɵĻ��������������O2���ȼ�պ�����ͨ��������Na2O2�����У�������������ӣ� ��
A��2��1 g B��3��6 g C��7��2 g D����ȷ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�껯ѧ�߿��ܸ�ϰ��ʱ���� 2-1���ʵķ�����ϰ���������棩 ���ͣ�ѡ����
��һ�������£����ᡢ��ζ��ܷ�����Ӧ�������ǣ� ��
A��K2O B��Na2CO3 C��CaCO3 D��CO2
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com