
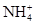 ��Cl��
��Cl�� ��
�� ����ȡ����100 mL��Һ��������ʵ�飺�ٵ�һ�ݼӹ���NaOH��Һ���Ⱥ�ֻ�ռ�������0.02 mol���������ɣ�ͬʱ�õ���Һ�ס����ڼ���Һ��ͨ�����CO2�����ɰ�ɫ���������������ˡ�ϴ�ӡ����գ�����Ϊ1.02 g���۵ڶ��ݼ�����BaCl2��Һ�ð�ɫ��������������������ϴ�ӡ����������Ϊ11.65 g����������ʵ��ش�
����ȡ����100 mL��Һ��������ʵ�飺�ٵ�һ�ݼӹ���NaOH��Һ���Ⱥ�ֻ�ռ�������0.02 mol���������ɣ�ͬʱ�õ���Һ�ס����ڼ���Һ��ͨ�����CO2�����ɰ�ɫ���������������ˡ�ϴ�ӡ����գ�����Ϊ1.02 g���۵ڶ��ݼ�����BaCl2��Һ�ð�ɫ��������������������ϴ�ӡ����������Ϊ11.65 g����������ʵ��ش�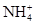 ��Al3����SO42-�������㣬
��Al3����SO42-�������㣬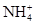 ��Al3�����ʵ�������0.02 mol��SO42-���ʵ�����0.05 mol..���ݵ���غ㣬K��һ������
��Al3�����ʵ�������0.02 mol��SO42-���ʵ�����0.05 mol..���ݵ���غ㣬K��һ������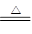 NH3����H2O�������ʵ���Ϊ0.02mol��ͬʱ��������˵��ԭ��Һ�в����ܺ���Mg2+
NH3����H2O�������ʵ���Ϊ0.02mol��ͬʱ��������˵��ԭ��Һ�в����ܺ���Mg2+ Al2O3��3H2O����Al2O3������Ϊ1.02 g���ɵ�ԭ��Һ��Al3�������ʵ���Ϊ0.02mol������Al3���Ĵ��ڣ�ͬʱ�ɷ���CO32-�Ĵ��ڣ�˫ˮ�ⷴӦ����
Al2O3��3H2O����Al2O3������Ϊ1.02 g���ɵ�ԭ��Һ��Al3�������ʵ���Ϊ0.02mol������Al3���Ĵ��ڣ�ͬʱ�ɷ���CO32-�Ĵ��ڣ�˫ˮ�ⷴӦ����

 �ܿ�����ĩ��̾�ϵ�д�
�ܿ�����ĩ��̾�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���Ȼ�����Һ | B��CH3Cl | C��������ˮ | D��Һ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��NH4+��Br����CO32�� | B��Na+��Br����CO32�� |
| C��Na+��I����SO32�� | D��NH4+��CO32����I�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| A��Cl2��H2O��HCl��HClO | B��2Na2O2��2H2O��4NaOH��O2�� |
| C��CaH2��2H2O��Ca(OH)2��2H2�� | D��3Fe��4H2O��g�� Fe3O4��4H2 Fe3O4��4H2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Na+��Ba2+��S2-��SO42- | B��H+��K+��SO32-��SO42- |
| C��Fe2+��H+��ClO-��S2- | D��K+��NH4+��SO42-��NO3- |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com