
ij��ѧ��ȤС���ú�����������ͭ�ĺϽ���ȡ�������Ȼ�����Һ���̷�����͵������壬��̽����ҵ���ϵ������á���ʵ�鷽�����£�
�Իش��������⣺
��1�������õ�����������ֽ������̨����Ȧ���ձ�����Ҫ����IJ���������_______________��
��2������ҺA�Ƶ�AlCl3��Һ��;����͢�����������Ϊ��������_____________��
��3������ҺE�еõ��̷������ʵ�������_______________________________��
��4��д��������F�Ʊ���������Ļ�ѧ����ʽ_____________________________��
��5����ͬѧ����ɽ�����������ܽ�Ͻ���ռ�������ᣬ������Ʒ�����Ҳ���Ƶ��������ʣ�����Ϊ���ߵķ����Ƿ��������________��������___________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��9�֣���ˮAlCl3��һ����Ҫ���л��ϳɴ�������������183��ʱ����������ʪ��������������������ij��ѧ��ѧ��ȤС����������ѧ�����������ʵ���Ʊ���ˮAlCl3��ʵ��װ������ͼ��ʾ��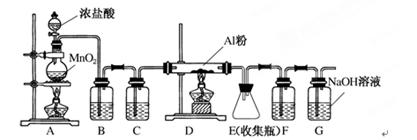
��ش��������⣺
��1���Ʊ�ʵ�鿪ʼʱ���ȼ��װ�õ������ԣ��������IJ���������________��
a������MnO2��ĩ b����ȼA�оƾ��� c������Ũ���� d����ȼD���ƾ���
��2��д��Aװ���з�����Ӧ�����ӷ���ʽ___________________________________��
��3��װ��B�е��Լ���__________����װ�ô��ڰ�ȫ��������ָ�� ��
��4����ͬѧ��ΪF��G������һ������������Ҽ���һ��ҩƷ���ɴﵽ��ͬЧ��������ҩƷ������____��
��5��E�еõ�������ɫ��ĩ������ľ��������Թ۲쵽��ƿ���а������ɣ��û�ѧ����ʽ��ʾ��ԭ��____________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�̲��и�����Na2O2��H2O��Ӧ�Ļ�ѧ����ʽ2Na2O2��2H2O=4NaOH��O2����Ϊ��̽��Na2O2��H2O��Ӧ�Ļ�����ijѧϰ̽��С���ڽ�ʦָ�����������ͼ��ʾװ�ý���ʵ�顣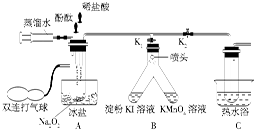
ʵ�鲽�����£�
�ٰ�ͼʾ��װ�����������װ��������Ϊ���ú�װ��ҩƷ��
�ڱ���K1��K2�رգ���ע�����е�����ˮ�����Թ��У���ʱ�Թ��в������������
�ۼ�ѹװ�з�̪�Ľ�ͷ�ιܣ�ʹ��̪�����Թ��У��Թ�����Һ�Ժ�ɫ��
�ܼ�ѹװ��ϡ����Ľ�ͷ�ιܣ�ʹϡ��������Թ��У���ɫ��ʧ���ٵμ�2�Ρ�
����˫����������A���Թ��й�����ʹ�Թ�����Һͨ����ͷ����B��֧���У����ֵ��ۣ�KI��Һ������KMnO4��Һ��ɫ��
��Ѹ�ٴ�K2���ر�K1��������A���Թ��й��������Թ�����Һ����C���Թ���Լ����֮һʱֹͣ������Ȼ������ˮԡ����C���Թ�Ƭ�̣�������ð����������Ϊ������
��ش��������⣺
��1�������ӷ���ʽ��ʾ���ۣ�KI��Һ������ԭ��_____________________________
________________________________________________________________________��
��2�������ӷ���ʽ��ʾKMnO4��Һ��ɫ��ԭ�� ______________________________
________________________________________________________________________��
��3��A���ñ�����ԡ��C������ˮԡ�����÷ֱ���________��______________________��
��4��Na2O2��H2O��Ӧ�Ļ�����____________________���û�ѧ����ʽ��ʾ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
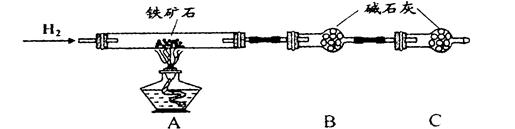
����ʯ�ǹ�ҵ��������Ҫԭ��֮һ������Ҫ�ɷ�Ϊ����������������в�����Ԫ�غ���Ԫ�أ������ʲ������ᷴӦ����ij�о���ѧϰС���ij����ʯ������������Ļ�ѧʽ����̽����
��.����ʯ�к������IJⶨ������ʵ����̲��������벹��������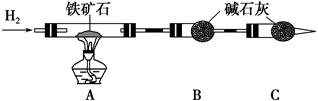
��1������ͼ��װ��������______________________________________________��
��2����8.0 g����ʯ����Ӳ�ʲ������У�װ��B��C�е�ҩƷ��ͼ��ʾ���г�������ʡ�ԣ���
��3������˵����ܿڴ����ϵػ���ͨ��H2��____________________________��
��ȼA���ƾ��ƣ�
��4����ַ�Ӧ�����ƾ��ƣ�________________________________________��
��5����÷�Ӧ��װ��B����2.25 g��������ʯ�����İٷֺ���Ϊ________��
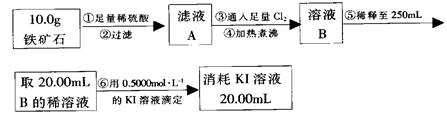
��.����ʯ�к������IJⶨ���������¡�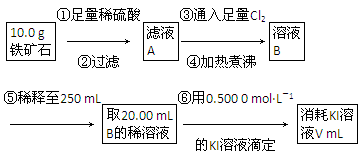
��1�����������������___________________________________________��
��2����������õ��IJ����������ձ�����ͷ�ιܡ�250 mL����ƿ��________��
��3�������йز���IJ�����˵����ȷ����________��
a����Ϊ��ˮΪ��ɫ�����Եζ������в����ָʾ��
b����ƿ����Ҫ�ô���Һ��ϴ
c���ζ������п����õ�����Һ��ָʾ��
d���ζ������У��۾�ע�ӵζ�����Һ��仯
e���ζ�������30 s����Һ���ָ�ԭ������ɫ���ٶ���
f���ζ������ζ��ܼ��첿�������ݣ���ⶨ���ƫ��
��4�����ζ�����������0.500 0 mol��L��1 KI��Һ20.00 mL��������ʯ�����İٷֺ���Ϊ________��
��.�ɢ���������������ʯ������������Ļ�ѧʽΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��.��������������Ԫ��,�̷�(FeSO4��7H2O)������ȱ����ƶѪҩƷ����Ҫ�ɷ֡�
(1)FeSO4��Һ�ڿ����л����������ʲ������ɫ����,�䷢����Ӧ�����ӷ���ʽ�� ;ʵ����������FeSO4��Һʱ������ �Է�ֹ�䱻�������������һ��ʵ��֤��FeSO4��Һ�Ƿ����� ��
��.���������[(NH4)2Fe(SO4)2��6H2O]�������������ױ���������,�����ڴ�������������
(2)��������鱗��ױ�������ԭ���� ��
(3)Ϊ����ֽ����ijɷ�,�������ʵ��װ�ý���ʵ��,����A�е�������������ֽ���ȫ��
��A�й����ּ��Ƚϳ�ʱ���,ͨ�뵪��,Ŀ���� ��
��װ��B��BaCl2��Һ��������Ϊ�˼���ֽ�������Ƿ���SO3��������,�����и�����,�۲쵽������Ϊ ��
��ʵ����,�۲쵽C���а�ɫ��������,��C�з����ķ�ӦΪ (�����ӷ���ʽ��ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��Ҫ�ⶨij�Ȼ��������Ȼ������������Ԫ�ص���������,�����²������ʵ��: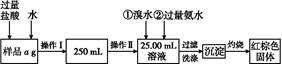
��.�������������,�ش���������:
��1�����������õ��IJ����������ձ�����������,�������������������������������������ƣ���
��2����д��������ˮ���������ӷ�Ӧ����ʽ: ����
��3�������������,��ȴ������,����ƽ����������Ϊb1 g,�ٴμ��Ȳ���ȴ�����³���������Ϊb2 g,��b1-b2="0.3" g,���������Ӧ���еIJ������� ��
��������������W1 g,����������Ⱥ������������W2 g,����Ʒ����Ԫ�ص���������������������������
��.��ͬѧ���,�����Բ������·������ⶨ: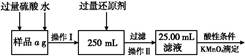
��1���ܽ���Ʒ����������,������������,Ϊʲô? ����
��2��ѡ��Ļ�ԭ���Ƿ�������������������ǡ���,ԭ����:�� ����
��3�����ζ��õ�c mol/L KMnO4��Һb mL,����Ʒ����Ԫ�ص���������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij�������д�����CuS���������������P��������������������ʡ�ij��ѧ����С������������̣��Ըÿ���Ϊԭ������CuCl2��2H2O���塣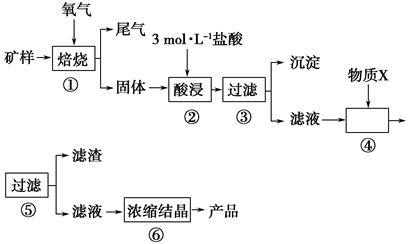
��֪����20 ��ʱ���Ȼ�ͭ���ܽ����73 g�������£��������ӿ�ʼ�����ͳ�����ȫʱ��pH���±���
| �������� | ��ʼ�γ��������������pH | ��ȫ�γ��������������pH |
| Fe2�� | 7.0 | 9.0 |
| Fe3�� | 1.9 | 3.2 |
| Cu2�� | 4.7 | 6.7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
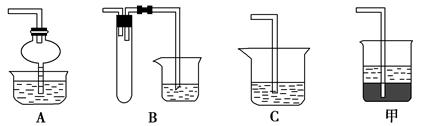
��15�֣�Ӳ�ʲ������ǻ�ѧʵ���о���ʹ�õ�һ�����������������ʵ�飨�̶�װ���ԣ����ش����⡣
������ʵ�飺��ͼ��ʾ����Ũ�������װ��Na2SO3�����������һ��ʱ���a��b��c��������仯���±�������д���еĿհף�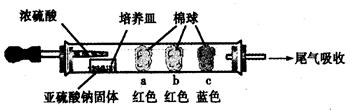
| ���� | �����ϵμӵ��Լ� | ʵ������ | ���ͺͽ��� |
| a | | �����ף��Ⱥ��ָֻ���ɫ | |
| b | ����̪��NaOH��Һ | �����Ϊ��ɫ | ���ӷ���ʽ�� |
| c | | �����Ϊ��ɫ | ��������� (ѡ������ԡ���ԭ�ԡ�) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijʵ��С����̽��Na2CO3��NaHCO3�����ʣ�����ʵ������ʢ�����ֹ�����Լ�ƿ��ʧ�˱�ǩ�����ǣ������ȶԹ���A��B���м�����ͨ��ʵ���������̽����
��1���ֱ���ȹ���A��B�����ֹ���A���Ȳ�����������ʹ����ʯ��ˮ����ǡ�A���ȷֽ�Ļ�ѧ��
��ʽΪ ��
��2����ȡ���ֹ����2 g���ֱ��������С�ձ��У��ٸ���10 mL ����ˮ���������¶ȱ仯����
�������ܽ⣬�ָ������£���������Һ�и�����2�η�̪��Һ��
�ٷ���Na2CO3������ȫ�ܽ⣬��NaHCO3������ʣ�࣬�ɴ˵ó����� ��
��ͬѧ�������ձ��л��۲쵽�����������У�ʢ��Na2CO3���ձ��г��ֵ������� ������ĸ��ţ���
a����Һ�¶��½� b����Һ�¶�����
c�������̪���dz��ɫ d�������̪��ʺ�ɫ
��3����ͼ��ʾ�������������õ�װ�â�͢��зֱ����ҩƷ���������ڵĹ���ͬʱ�����Թ��С�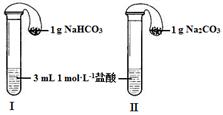
�����Թ��о��������壬 ������ķ�Ӧ�̶ȸ�Ϊ���ҡ�
�ڷ�Ӧ����������������ͣ��ָ������£�����˵����ȷ���� ��
a��װ�â����������ϴ� b��װ�â����������ϴ�
c������������������������� d�����������������ݹ������
��4��ͬѧ�ǽ����ֹ���ֱ����Ƴ�0.5 mol��L-1����Һ��������·������Է�Ӧ��������Ԥ�⣺
| ʵ�鷽�� | Ԥ������ | Ԥ������ |
| ����1����2 mL Na2CO3��Һ�еμ�1 mL 0.5 mol��L-1CaCl2��Һ | �а�ɫ ���� | Na2CO3��Һ�е�CO32-Ũ�Ƚϴ�����CaCl2������Ӧ ��д���ӷ���ʽ���� |
| ����2����2 mL NaHCO3��Һ�еμ�1 mL 0.5 mol��L-1CaCl2��Һ | �ް�ɫ ���� | NaHCO3��Һ�е�CO32-Ũ�Ⱥ�С��������CaCl2��Ӧ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com