
��1��P4�����ף�s����5O2��g���T�T P4O10��s�� ��H����2983.2kJ/mol
P�����ף�s����5/4O2��g���T�T 1/4 P4O10��s�� ��H����738.5kJ/mol
�����ת��Ϊ�����Ȼ�ѧ����ʽΪ ��
��ͬ״���£������ȶ��ԱȰ��� ���ǿ���������������жϡ���
��2����ԭ����У�ͨ���ϻ��õĽ����� �������� ��Ӧ��
�����У����Դ���������ļ��� �������� ��Ӧ��
��3����ͼ��ʾˮ�����Թ�����һö���������������۲죺
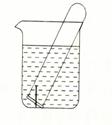
�����Թ���Һ������������ ��ʴ��������ӦʽΪ ��
�����Թ���Һ���½������� ��ʴ��������ӦʽΪ ��
��1��P4�����ף�s����4P�����ף�s�� ��H����29.2 kJ/mol��2�֣�
ǿ ��1�֣� ��2���� �� ���� �� �� �� ���� ����1�֣�
��3������ ��1�֣� O2+4e-+2H2O��4OH- ��2�֣�
���� ��1�֣� 2H++2e-��H2 �� ��2�֣�
��������
�����������1�����ݸ�˹���ɿ�֪���٣��ڡ�4���õ�P4�����ף�s����4P�����ף�s�������Ը÷�Ӧ�ķ�Ӧ�Ȧ�H����2983.2kJ/mol��738.5kJ/mol��4����29.2 kJ/mol����˵���������������ں��������������Ժ����ȶ���ǿ�ڰ��ġ�
��2����ԭ����У�ͨ���ϻ��õĽ�����������ʧȥ���ӣ�����������Ӧ���ڵ���У����Դ���������ļ���������ʧȥ���ӣ�����������Ӧ��
��3�������Թ���Һ��������˵���Թ���ѹǿ��С�����������ģ������������ʴ��������Ӧʽ��O2+4e-+2H2O��4OH-��
�����Թ���Һ���½���˵���Թ���ѹǿ�������������ɣ���������ⸯʴ��������Ӧʽ��2H++2e-��H2����
���㣺�����˹���ɵ�Ӧ�á��绯ѧԭ�����ж��Լ������绯ѧ��ʴ���й��ж�
�����������ǻ���������Ŀ��飬�ѶȲ�����Ҫ�ǿ���ѧ���Ի�������֪ʶ���˽�����������Լ�������û���֪ʶ���ʵ�����������������Ĺؼ������պõ绯ѧԭ���Լ��йص�Ӧ�ã�����������ѧ������˼ά������Ӧ��������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(6��)��1��P4�����ף�s����5O2��g����P4O10��s�� ��H����2983.2kJ/mol
��2��P�����ף�s����5/4O2��g����1/4 P4O10��s�� ��H����738.5kJ/mol
�����ת��Ϊ�����Ȼ�ѧ����ʽΪ___________________����ͬ״���£������ϵ͵���______�������ȶ��ԱȰ���____
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ������ʡ��ʦ���и߶���ѧ����ĩ���⻯ѧ�������Ծ� ���ͣ������
(6��)��1��P4�����ף�s����5O2��g����P4O10��s�� ��H����2983.2kJ/mol
��2��P�����ף�s����5/4O2��g����1/4 P4O10��s�� ��H����738.5kJ/mol
�����ת��Ϊ�����Ȼ�ѧ����ʽΪ___________________����ͬ״���£������ϵ͵���______�������ȶ��ԱȰ���____
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013���������������ʮ����ѧ�߶���ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ������
��1��P4�����ף�s����5O2��g���T�T P4O10��s�� ��H����2983.2kJ/mol
P�����ף�s����5/4O2��g���T�T 1/4 P4O10��s�� ��H����738.5kJ/mol
�����ת��Ϊ�����Ȼ�ѧ����ʽΪ ��
��ͬ״���£������ȶ��ԱȰ��� ���ǿ���������������жϡ���
��2����ԭ����У�ͨ���ϻ��õĽ����� �������� ��Ӧ��
�����У����Դ���������ļ��� �������� ��Ӧ��
��3����ͼ��ʾˮ�����Թ�����һö���������������۲죺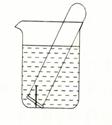
�����Թ���Һ������������ ��ʴ��������ӦʽΪ ��
�����Թ���Һ���½������� ��ʴ��������ӦʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013������ʡ�߶���ѧ����ĩ���⻯ѧ�������Ծ� ���ͣ������
(6��)��1��P4�����ף�s����5O2��g����P4O10��s�� ��H����2983.2kJ/mol
��2��P�����ף�s����5/4O2��g����1/4 P4O10��s�� ��H����738.5kJ/mol
�����ת��Ϊ�����Ȼ�ѧ����ʽΪ___________________����ͬ״���£������ϵ͵���______�������ȶ��ԱȰ���____
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com