
������Ѫ�쵰����Ҫ��ɳɷ֣�������������֯����O2�����ã����ȱ���Ϳ��ܳ���ȱ����ƶѪ�����������������Ҳ�к���������һ�ֳ�����ҩƷ˵�����еIJ������ݣ���ҩƷ��Fe2+33%��36%��������ˮ�������������е�θ���Vc��ά����C��ͬ�������ӱ�Ʒ���ա�
��һ����ͬѧ�����������ʵ����ò���ҩƷ���Ƿ���Fe2+��̽��Vc�����ã�
��1������������ˮ����Һ�з��������ӷ�Ӧ����ʽ��_________________________��
Fe3+ +SCN-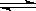 [Fe(SCN) ]2+��
[Fe(SCN) ]2+��
��2������KSCN��Һ����Һ��Ϊ����ɫ��˵����Һ��������Fe3+�������Ӵ��ڵ�ԭ������ǣ����ţ�_____________________��
A��ҩƷ�е���������Ӧ��������������ʽ����
B������ҩ��������������������
C��ҩƷ�������������������������
��3��ҩƷ˵�����С���Vcͬ�������ӱ�Ʒ���ա���˵������_______________________��
��������ͬѧ�����������������ø�����ر���Һ�ζ��ķ����ⶨ��ҩƷ�Ƿ�ϸ�Ӧԭ��Ϊ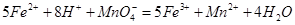 ��ȷ��������ҩƷ10.00g������ȫ�������Լ�2�У����Ƴ�1000mL��Һ��ȡ��20.00mL����0.0200mol/L��KMnO4��Һ�ζ�����ȥKMnO4��Һ12.00mL��
��ȷ��������ҩƷ10.00g������ȫ�������Լ�2�У����Ƴ�1000mL��Һ��ȡ��20.00mL����0.0200mol/L��KMnO4��Һ�ζ�����ȥKMnO4��Һ12.00mL��
��4����ʵ���е��Լ�2���ͬѧ��Ƶ�ʵ���е��Լ�1��������______������ţ���
A������ˮB��ϡ����C��ϡ����D��ϡ����
��5����ʵ��ζ������в����ζ��ܵ�ͼʾ��ȷ����_______�����ţ���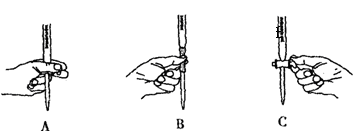
��6����ͨ�����㣬˵����ҩƷ�����������Ƿ�ϸ�д����Ҫ������̣���
��1�� ��2��B��C
��2��B��C
��3��Fe2+������Ѫ�쵰��������O2�����ã�Fe3+û�д˹��ܣ�����Vc�ɷ�ֹҩƷ�е�Fe2+��������Fe3+���������ֻ������Fe2+��Fe2+������Ѫ�쵰��������O2�����ã�����Vc�ɷ�ֹҩƷ�е�Fe2+��������Fe3+��������������Ҳ�ɸ��֣���
��4��C��5��A
��6��

����������Ϊ�� ,��ҩƷ�����������ϸ��ֲ��ʵ����֣�
,��ҩƷ�����������ϸ��ֲ��ʵ����֣�
���������������һ����1�����������Ϣ������ͼ֪������������ˮ����Һ��Ѫ��ɫ��Fe2+��������Fe3+�����������ӷ�Ӧ����ʽ��Cl2 + 2Fe2+=2Fe3+ + 2Cl-��Fe3+ +SCN-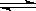 [Fe(SCN) ]2+����2������KSCN��Һ����Һ��Ϊ����ɫ��˵����Һ��������Fe3+��A�����������Ϣ֪ҩƷ�е���Ӧ���Զ���������ʽ���ڣ�����B�����������н�ǿ�Ļ�ԭ�ԣ�����ҩ�����п��ܱ������е���������������������������ȷ��C�����������н�ǿ�Ļ�ԭ�ԣ�ҩƷ��������п��ܱ������е���������������������������ȷ��ѡBC����3���������Ϣ֪��Vc���л�ԭ�ԣ�Fe2+������Ѫ�쵰��������O2�����ã�Fe3+û�д˹��ܣ�����Vc�ɷ�ֹҩƷ�е�Fe2+��������Fe3+���������ֻ������Fe2+��Fe2+������Ѫ�쵰��������O2�����ã�����Vc�ɷ�ֹҩƷ�е�Fe2+��������Fe3+������������4�����������Ϣ֪����ҩƬ�������ᣬ��������������ر���Һ����������ԭ��Ӧ��ϡ�������ǿ�����ԣ��ܽ�Fe2+������Fe3+����ѡ��ϡ���ᣬѡC����5����ʵ��ζ��������õ�������ر���Һ����ǿ�����ԣ�Ӧ����ʽ�ζ���ʢװ���ζ�ʱӦ���ֿ��ƻ���������ҡ����ƿ�������ζ��ܵ�ͼʾ��ȷ����A����6�����������ѧ����ʽ�ù�ϵʽ��5Fe2+����MnO4������
[Fe(SCN) ]2+����2������KSCN��Һ����Һ��Ϊ����ɫ��˵����Һ��������Fe3+��A�����������Ϣ֪ҩƷ�е���Ӧ���Զ���������ʽ���ڣ�����B�����������н�ǿ�Ļ�ԭ�ԣ�����ҩ�����п��ܱ������е���������������������������ȷ��C�����������н�ǿ�Ļ�ԭ�ԣ�ҩƷ��������п��ܱ������е���������������������������ȷ��ѡBC����3���������Ϣ֪��Vc���л�ԭ�ԣ�Fe2+������Ѫ�쵰��������O2�����ã�Fe3+û�д˹��ܣ�����Vc�ɷ�ֹҩƷ�е�Fe2+��������Fe3+���������ֻ������Fe2+��Fe2+������Ѫ�쵰��������O2�����ã�����Vc�ɷ�ֹҩƷ�е�Fe2+��������Fe3+������������4�����������Ϣ֪����ҩƬ�������ᣬ��������������ر���Һ����������ԭ��Ӧ��ϡ�������ǿ�����ԣ��ܽ�Fe2+������Fe3+����ѡ��ϡ���ᣬѡC����5����ʵ��ζ��������õ�������ر���Һ����ǿ�����ԣ�Ӧ����ʽ�ζ���ʢװ���ζ�ʱӦ���ֿ��ƻ���������ҡ����ƿ�������ζ��ܵ�ͼʾ��ȷ����A����6�����������ѧ����ʽ�ù�ϵʽ��5Fe2+����MnO4������ ��
�� ��������������
������������Ϊ�� ,��ҩƷ�����������ϸ�
,��ҩƷ�����������ϸ�
���㣺�������ӷ���ʽ����д����Ԫ�ػ���������ʾ͡�������ԭ�ζ�����ϵʽ�����㣬�����Ķ����Ͻ�����Ϣ�����������������ѧ֪ʶ��������


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ͭ��ʯ����CuO��Cu2(OH) 2CO3��������Fe2O3��FeO��SiO2�ȡ�ͭ�������������������Ԫ�ء�ij���ϳ�������������ͭ���������������������£�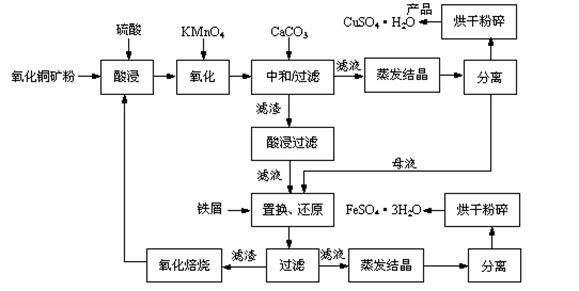
��1������ͭ��ʯ�����Ŀ���� ��
��2��д�����������Cu2(OH) 2CO3������Ӧ�����ӷ���ʽ ��
��3�����к�/���ˡ��м���CaCO3��Ŀ���� ��
��4���������������жദ�漰�����ˡ���ʵ�����й��˲�����Ҫʹ�õIJ��������� ��
��5��������������� �� ��
��6���±�����ͼΪ����ɷ��顱����������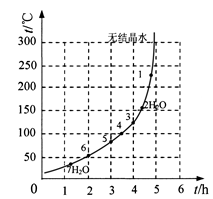
����������������ͼ
| ��� | t/h | t/�� | m/g | x |
| 1 | 3 | 80 | 5 | 4 |
| 2 | 3 | 160 | 10 | 1 |
| 3 | 4 | 160 | 10 | 0.8 |
| 4 | 4 | 160 | 5 | 0 |
| 5 | 5 | 80 | 5 | 3 |
| 6 | 5 | 160 | 15 | 1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(����Ԥ����)(1)���ڵ��۵⻯����Һ�У��μ������������Ƽ�����Һ�������ῴ����Һ����ɫ��������Ϊ________�����ӷ���ʽΪ__________________________��
���ڵ�͵����γɵ���ɫ��Һ�У��μ��������Ƽ�����Һ��������ɫ����ʧ��������Ϊ______________________________�����ӷ���ʽ��_______________________________��
�۶ԱȢٺ͢�ʵ�����õĽ������I2��ClO����SO42������ǿ������˳������Ϊ_____________________________��
(2)������Ƭ��ͭƬ�����ʵ��֤��������ʵ����д����Ӧ�Ļ�ѧ����ʽ��
��Ũ����������Ա�ϡ����ǿ��________________________________��
���Ȼ�����Һ��Fe3���������Ա�����ͭ��Һ�е�Cu2��ǿ��__________________________��
�����Ļ�ԭ�Ա�ͭǿ��
___________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij��Һ���ܺ���Na����K����Mg2����Cu2���������Ӽ�MnO4����SiO32����AlO2����CO32����HCO3����SO42����Cl���������ӣ���֪���ٸ���Һ����ɫ���ھ��ⶨ��Һ��pH��12����ȡ������Һ������100 mL 2 mol��L��1ϡ��������ữ���а�ɫ�������ɣ����õ�һ����ɫ��ζ�����壬������ʹ����ʯ��ˮ(����)����ǡ����ữ�����Һ���ˣ��õ���Һ�ס�
(1)�ɢ٢ڢۿ��жϣ�ԭ��Һ��һ�������ڵ�������________��һ�����ڵ�������________��
(2)����Һ�ֳ����ȷݣ�һ������μ��백ˮ�������а�ɫ��״������˵��ԭ��Һ��һ����________(�����ӷ���)���տ�ʼ���백ˮʱ��û�г���������ԭ����____________________________________(�����ӷ���ʽ��ʾ)����һ���м���������Ba(NO3)2��Һ���а�ɫ�������ɣ�˵��ԭ��Һ��һ����________(�����ӷ���)�����˵õ���Һ�ҡ�
(3)����Һ���м���������AgNO3��Һ�����ˡ�ϴ�ӡ�����ù���26.5 g����ԭ��Һ���Ƿ���Cl����________(��ǡ���)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪ 0.4 mol Һ̬�º�����H2O2��Ӧ���ɵ�����ˮ����ʱ�ų�256.64 kJ��������
(1)д���º�H2O2��Ӧ���Ȼ�ѧ����ʽ: ��
(2)��֪H2O(l)=H2O(g) ��H="+44" kJ/mol,��16 gҺ̬��������˫��ˮ��Ӧ���ɵ�����Һ̬ˮʱ,�ų��������� ��
(3)������ӦӦ���ڻ���ƽ���,���ͷų����������Ϳ��ٲ�������������,����һ����ͻ�����ŵ��� ��
(4)�����������Һ��ͨ��һ�����ʵ����İ�����������,д����Ӧ�����ӷ���ʽ: ,�÷�Ӧ�Ļ�ԭ������ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ۺ�����ת¯ú��[CO��60��80%����CO2��15��20%������N2��]�����Ṥҵβ���е�SO2�����ܾ���β�������ܻ�ñ��շۣ�Na2S2O4�����䲿�ֹ����������£�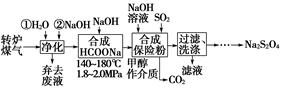
��1��ת¯����ʱ�����ڷ�Ӧ��Fe3C��s����CO2��g��??2CO��g����3Fe��s������ƽ�ⳣ������ʽΪK��________��
��2��ú������ʱ������ˮϴ����NaOH��Һϴ�ӣ���Ŀ����________��
��3������Һ�л��ռ״��IJ���������____________________________��
���ɻ��յ�����������______________________________________��ֻдһ�ֻ�ѧʽ����
��4���ϳɱ��շ۷�Ӧ�Ļ�ѧ����ʽΪ_________________________��
��5�����շۡ�H2O2��������ֽ��Ư�ף�д�����շ��������H2O2����ˮ��Һ�з�Ӧ���������ε����ʵ����ӷ���ʽ��________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������ֿ���������A��B��C��D��E�������������������ӻ�����ͬ���ֱ�������������Na����Al3����Mg2����Ba2����Fe3��������������Cl����OH����NO3����CO32����X�е�һ�֡�
(1)ijͬѧͨ���ȽϷ�������Ϊ�������Ϳ��ж����б��е�����������________��________(�ѧʽ)��
(2)Ϊ��ȷ��X���ֽ�(1)�е��������ʼ�ΪA��B����X�����ʼ�ΪC����C��B��Һ���ʱ���������ɫ��������ɫ��ζ���壻��C��A����Һ���ʱ�����ػ�ɫ��������ó����е���ϡ������������ܽ⣬������а�ɫ���������ܽ⡣��XΪ________��
A��SO32����������B��SO42�� C��CH3COO�� D��SiO32��
(3)��CuͶ�뵽װ��D��Һ���Թ��У�Cu���ܽ⣻�ٵμ�ϡH2SO4��Cu���ܽ⣬�ܿڸ����к���ɫ������֡�������Dһ���������������е�________(����Ӧ�����ӷ���)���йط�Ӧ�����ӷ���ʽΪ___________________��
(4)���������Ѿ�ȷ�������ʣ����Լ����D��E�е������ӣ������ʵ��������衢�����ۣ�__________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����������ʣ���NaCl���壻��Һ̬SO2���۴���������ᱵ������ͭ���ƾ���C2H5OH�������ۻ���KCl����NaOH��Һ��
�����������ʻش��������⡣������ţ�
��1��������״̬���ܵ��������������������������
��2������������ʵ�������������������
��3�����ڷǵ���ʣ�������ˮ���ˮ��Һ�ܵ��������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ijNa2CO3��NaAlO2�Ļ����Һ����μ���1 mol��L-1������,�����Һ�е�C ��HC
��HC ��Al
��Al ��Al3+�����ʵ�����������������仯��ϵ��ͼ��ʾ,������˵����ȷ���ǣ� ��
��Al3+�����ʵ�����������������仯��ϵ��ͼ��ʾ,������˵����ȷ���ǣ� ��
A��ԭ�����Һ�е�C ��Al ��Al �����ʵ���֮��Ϊ1��2 �����ʵ���֮��Ϊ1��2 |
| B��V1��V2=1��5 |
| C��M��ʱ���ɵ�CO2Ϊ0.05 mol |
D��a�߱�ʾ�����ӷ���ʽΪ:Al +H++H2O +H++H2O Al(OH)3�� Al(OH)3�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com