
��14�֣���Ч��ˮ���ۺ��Ȼ�������PAFC������ɿɱ�ʾΪ[AlFe(OH)nCl6-n]m�������ʹ㷺Ӧ����
�ճ�������ˮ ��ҵ��ˮ�Ĵ�����
��ҵ��ˮ�Ĵ�����
��1��Ϊ���PAFC��Al��Fe������������ͼ��ʾ���̽��С�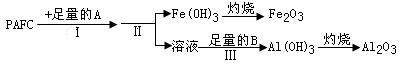
�ش��� �����⣺
�����⣺
��PAFC����Ԫ�صĻ��ϼ�Ϊ_________��
�ڲ���I�е��Լ�A��______ ___�����ˮ��������������Һ������
�۲����������� ��
�ܲ��������ӷ���ʽΪ_________________________________ __��
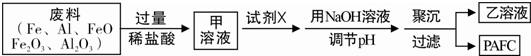
��2��ij�������Թ�ҵ���ϣ��������������������Ϊԭ����ȡPAFC������������̣�
������������Һ�У�����ȷ���Ƿ�һ�����ڵ���������___________
A��Al3+ B��Fe2+ C��Fe3+ D��H+
Ϊ֤��������ȷʵ���ڣ��ɲ��õ��Լ���_____________________��
�������� ��Һ�м����Լ�X��Ŀ����_________________________________��
��Һ�м����Լ�X��Ŀ����_________________________________��
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
 Al��OH��3+3H+
Al��OH��3+3H+ Al��OH��3+3H+
Al��OH��3+3H+�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��PAFC��ˮ����ܾ�ˮ | B��������Һ�е���KSCN��Һ���Ѫ��ɫ | C���Լ�X���������� | D������Һ�϶������ܳ�ǿ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
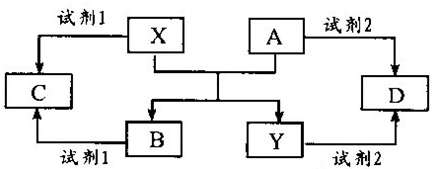
A��B��C��D��E����ѧ������5�ֻ����A��B�������
X��Y�������г����Ľ������ʣ�������ʼ�Ĺ�ϵ����ͼ��ʾ��

��ش��������⣺
(1) д��X��A��Ӧ�Ļ�ѧ����ʽ��___________________��
(2) ���Լ�l��NaOH��Һ��д��X���Լ�1��Ӧ�����ӷ���ʽ___________________ ��
(3)���Լ�1���Լ�2����ϡ���ᡣ
�ټ�������D����Һ�н������ӵķ�����___________________ ��
�ڽ�����C����ˮ������Һ�����ԣ�ԭ����___________________ (�����ӷ���ʽ��ʾ)��
��ij��Ч��ˮ������Y(OH)SO4�ۺϵõ��ġ���ҵ����D��ϡ�������������Ϊԭ�����Ʊ�Y(OH)SO4����Ӧ����NO���ɣ��÷�Ӧ�Ļ�ѧ����ʽ��___________________ ��
(4)���Լ�1���Լ�2����ϡ���ᣬ��C��D��Ϻ�ͨ������Z���ٵμ�����������Һ������Һ��pH����ˮ��ۺϵõ���Ч��ˮ���ۺ��Ȼ�����[AlFe(OH)nCl6-n]m������Z��__________����������
________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com