
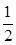 O2��g��
O2��g�� SO3��g����H����98 kJ��mol��1��
SO3��g����H����98 kJ��mol��1��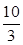 �����ڴ��¶��£���100 L�ĺ����ܱ������У�����3.0 mol
�����ڴ��¶��£���100 L�ĺ����ܱ������У�����3.0 mol
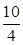 ��K`��K���ʷ�Ӧ������Ӧ�����ƶ�������Ӧ���ʣ��淴Ӧ���ʡ�
��K`��K���ʷ�Ӧ������Ӧ�����ƶ�������Ӧ���ʣ��淴Ӧ���ʡ�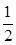 O2��g��
O2��g�� SO3��g�������Ϊ2 L���ܱ������г���2.0 mol SO2��1.0
SO3��g�������Ϊ2 L���ܱ������г���2.0 mol SO2��1.0 SO3��g��
SO3��g��

 ��ĩ100�ִ��غ�������ϵ�д�
��ĩ100�ִ��غ�������ϵ�д� Сѧ�������Ծ�ϵ�д�
Сѧ�������Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���


 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
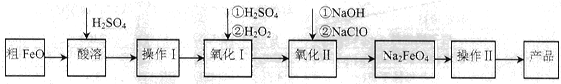
| �¶�/�� | 15 | 20 | 25 | 30 | 35 | 40 | 45 |
| NaClOŨ��/mol��L-1 | 4.6 | 5.2 | 5.4 | 5.5 | 4.5 | 3.5 | 2 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
| T/K | 303 | 313 | 323 | 353 |
| NH3������/(10��6 mol) | 4.8 | 5.9 | 6.0 | 2.0 |
 4NH3(g)��3O2(g)��H��a kJ��mol��1
4NH3(g)��3O2(g)��H��a kJ��mol��1 2NH3(g)����H����92 .4 kJ��mol��1
2NH3(g)����H����92 .4 kJ��mol��1 2NH3(g)����H����92 .4 kJ��mol��1���ֱ��о���T1��T2��T3(T1<T2<T3)�����¶��ºϳɰ����Ĺ��ɡ���ͼ�����������¶��²�ͬ��H2��N2����ʼ��ɱ�(��ʼʱN2�����ʵ�����Ϊ1 mol)��N2ƽ��ת���ʵĹ�ϵ����ش�
2NH3(g)����H����92 .4 kJ��mol��1���ֱ��о���T1��T2��T3(T1<T2<T3)�����¶��ºϳɰ����Ĺ��ɡ���ͼ�����������¶��²�ͬ��H2��N2����ʼ��ɱ�(��ʼʱN2�����ʵ�����Ϊ1 mol)��N2ƽ��ת���ʵĹ�ϵ����ش�
 N2(g)��3H2(g)��ƽ�ⳣ��Ϊ________ ��
N2(g)��3H2(g)��ƽ�ⳣ��Ϊ________ ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
 CO(g)��H2(g)��
CO(g)��H2(g)�� H2(g)��CO2(g)��
H2(g)��CO2(g)�� CO2(g)��H2(g)�ﵽ��ѧƽ��״̬��������________��(��ѡ��©ѡ����ѡ�����÷�)
CO2(g)��H2(g)�ﵽ��ѧƽ��״̬��������________��(��ѡ��©ѡ����ѡ�����÷�) 2NH3(g)����H��0�����ѹǿ��ʱ��ͼ����ͼ�ף����p2��0.6p1����ʱ�¶�����ʼ�¶���ͬ���ڴﵽƽ��ǰijһʱ��(t1)�����ı�һ���������õ�����ͼ��
2NH3(g)����H��0�����ѹǿ��ʱ��ͼ����ͼ�ף����p2��0.6p1����ʱ�¶�����ʼ�¶���ͬ���ڴﵽƽ��ǰijһʱ��(t1)�����ı�һ���������õ�����ͼ��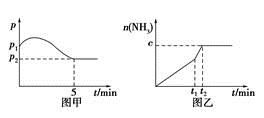
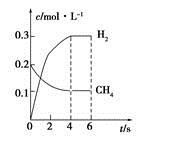
 CO(g)��3H2(g)�Ʊ�CO��H2����һ��������1 L���ܱ������г���0.3 mol H2O��0.2 mol CH4�����H2(g)��CH4(g)�����ʵ���Ũ����ʱ��仯��������ͼ��ʾ��0��4 s�ڣ���CO(g)��ʾ�ķ�Ӧ����Ϊ____________��
CO(g)��3H2(g)�Ʊ�CO��H2����һ��������1 L���ܱ������г���0.3 mol H2O��0.2 mol CH4�����H2(g)��CH4(g)�����ʵ���Ũ����ʱ��仯��������ͼ��ʾ��0��4 s�ڣ���CO(g)��ʾ�ķ�Ӧ����Ϊ____________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
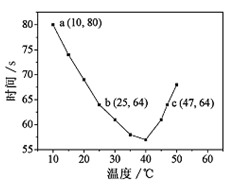
| A��40��֮ǰ��40��֮����Һ������ʱ�����¶ȵı仯�����෴ |
| B��ͼ��b��c�����Ӧ��NaHSO3��Ӧ������� |
| C��ͼ��a���Ӧ��NaHSO3��Ӧ����Ϊ5.0 ��10-5mol��L-1��s-1 |
| D���¶ȸ���40��ʱ�����۲��������������ָʾ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 2NH3�ﵽ��ѧƽ��״̬������˵��һ����ȷ����
2NH3�ﵽ��ѧƽ��״̬������˵��һ����ȷ����| A��ÿ1 mol N��N���ѵ�ͬʱ��2 mol N��H���� |
| B��N2��H2��NH3��Ũ��֮��Ϊ1:3:2 |
| C��N2���ٵ����ʺ�NH3���ٵ�����֮��Ϊ1:2 |
D���������Ϊ��ʼ�����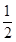 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 xC(g)��D(g)��5min��Ӧ�ﵽƽ�⣬������ѹǿ��С�����D��ƽ����Ӧ����Ϊ0.05mol?L-1?min-1 �����н��۴������( )
xC(g)��D(g)��5min��Ӧ�ﵽƽ�⣬������ѹǿ��С�����D��ƽ����Ӧ����Ϊ0.05mol?L-1?min-1 �����н��۴������( ) | A��A��ƽ����Ӧ����Ϊ0.15 mol?L-1?min-1 |
| B��ƽ��ʱ��C��Ũ��Ϊ0.25mol?L-1 |
| C��B��ƽ����Ӧ����Ϊ0.1 mol?L-1?min-1 |
| D��ƽ��ʱ��������ѹǿΪԭ����0.875�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 H2����I2
H2����I2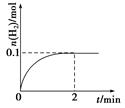
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com